Preparation method of hydrochloric acid cyclobenzaprine
A technology of cyclobenzaprine hydrochloride and alcohol hydrochloride, applied in the field of medicine and chemical industry, can solve the problems of cumbersome process, large amount of high-salt wastewater, time and energy consumption of concentrated solvents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The embodiment of the present invention discloses a preparation method of cyclobenzaprine hydrochloride, comprising the following steps:
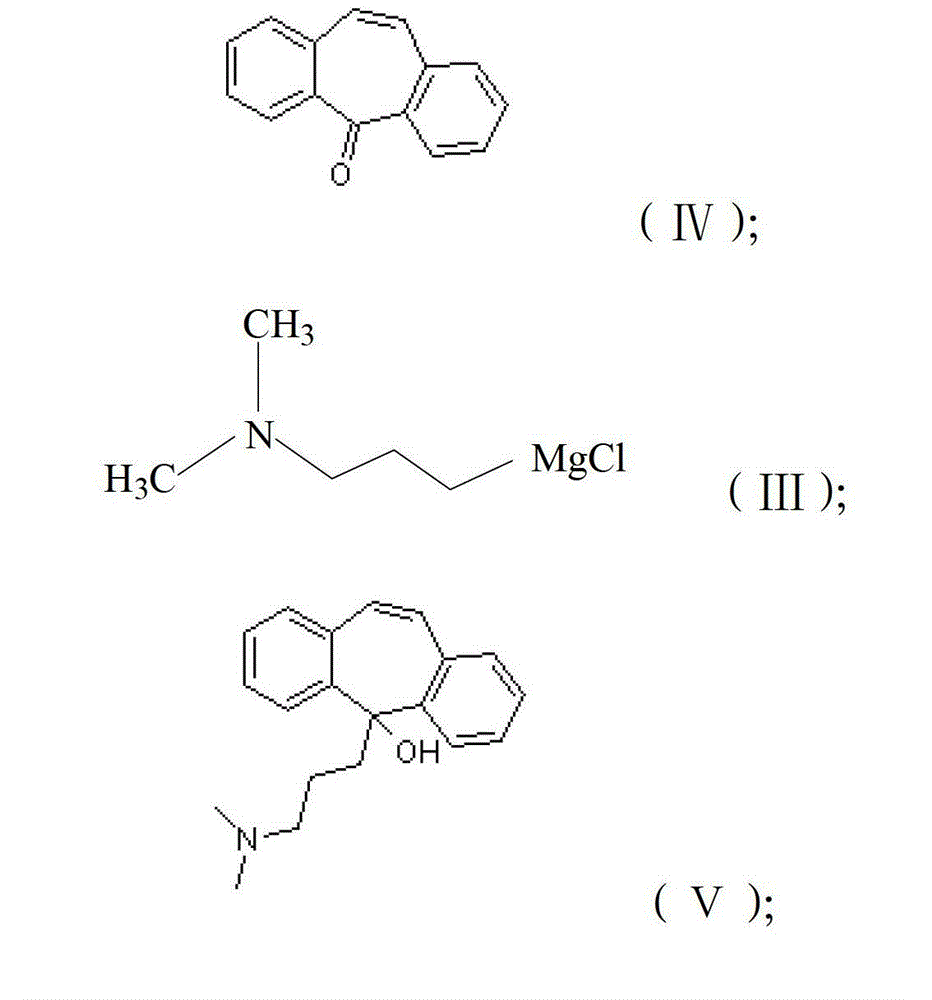
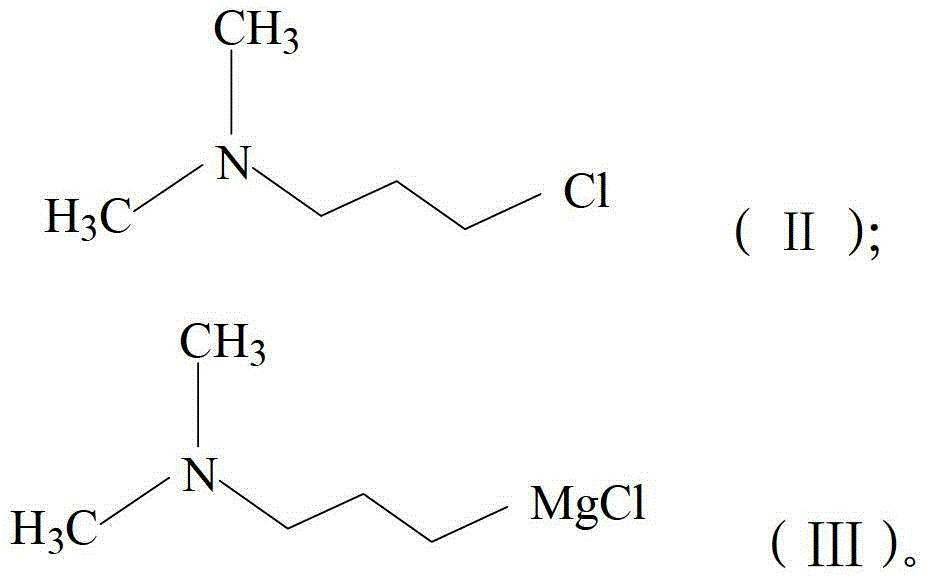
[0025] a) Reaction of dibenzocyclohepten-5-one of formula (IV) with N,N-dimethyl-3-propylamine magnesium chloride of formula (III) in a solution of tetrahydrofuran and toluene , to obtain 5-(3-(dimethylamino)propyl)-5H-dibenzo[a,d]cyclohepten-5-ol as shown in (V);
[0026] b) reacting one of hydrogen chloride gas or hydrogen chloride alcohol solution with the 5-(3-(dimethylamino)propyl)-5H-dibenzo[a,d]cyclohepten-5-ol to obtain 5-(3-(Dimethylamino)propyl)5H-dibenzo[a,d]cyclohepten-5-ol hydrochloride as (VII);
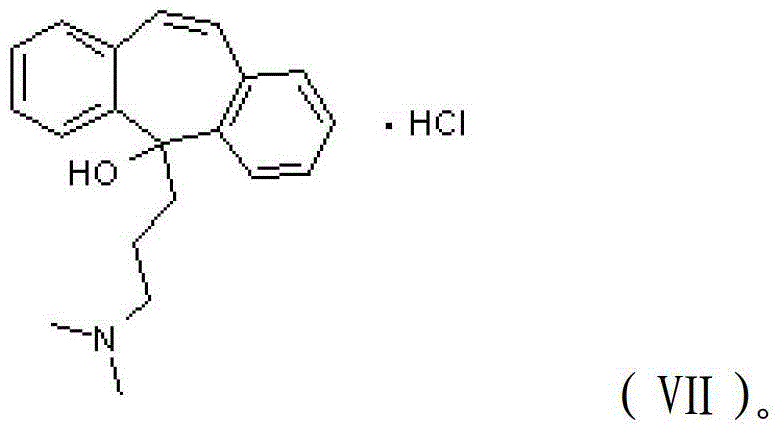
[0027] c) dehydrating the 5-(3-(dimethylamino)propyl)-5H-dibenzo[a,d]cyclohepten-5-ol hydrochloride to obtain cyclobenzaprine hydrochloride.
[0028] According to the present invention, the reaction formula of preparing described cyclobenzaprine hydrochloride is shown in the following formula:
[0029]
[0030] The prepa...
Embodiment 1
[0044] A 1000mL dried glass-jacketed reaction kettle was added under nitrogen protection with 11.77g (484mmol) of metal magnesium chips, 80mL of anhydrous tetrahydrofuran (THF) and 12mL of a toluene solution of N,N-dimethyl-3-chloropropylamine, in which N , the content of N-dimethyl-3-chloropropylamine is 25wt%, and 0.2g of iodine particles are added, after initiating the reaction, a total of 49.7g (404mmol) of N,N-dimethyl-3-chloropropylamine ( II) in toluene solution, and then stirred at 80-100°C for 1 hour to obtain N,N-dimethyl-3-propylamine magnesium chloride toluene / THF solution.
[0045] 75g (364mmol) of dibenzocyclohepten-5-one and 340ml of toluene were added dropwise to the toluene / THF solution of N,N-dimethyl-3-propylamine magnesium chloride at a temperature below 35°C, and stirred for 1 ~4 hours, detected by HPLC; 80 g of saturated ammonium chloride solution was added dropwise to obtain a solid-containing mixed solution, which was then filtered, and 20 g (544 mmol) ...
Embodiment 2
[0048] The preparation of N,N-dimethyl-3-propylamine magnesium chloride toluene / THF solution is the same as in Example 1. 75g (364mmol) of dibenzocyclohepten-5-one and 340ml of toluene were added dropwise to the toluene / THF solution of N,N-dimethyl-3-propylamine magnesium chloride at a temperature below 35°C, and stirred for 1 ~4 hours, HPLC detection; slowly add 120g of saturated ammonium chloride solution dropwise to obtain a mixed solution containing solids, filter, and pass the filtrate into 100g of isopropanol solution containing 20g (544mmol) of hydrogen chloride, after passing through, stir for 1 hours, filtered to obtain 106 g of 5-(3-(dimethylamino)propyl)-5H-dibenzo[a,d]cyclohepten-5-ol hydrochloride, which was directly used in the next step without drying.
[0049] 5-(3-(Dimethylamino)propyl)-5H-dibenzo[a,d]cyclohepten-5-ol hydrochloride was heated to 80 °C with 700 mL of isopropanol and stirred for 1 h, monitored by HPLC Reaction, when 5-(3-(dimethylamino)propyl)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com