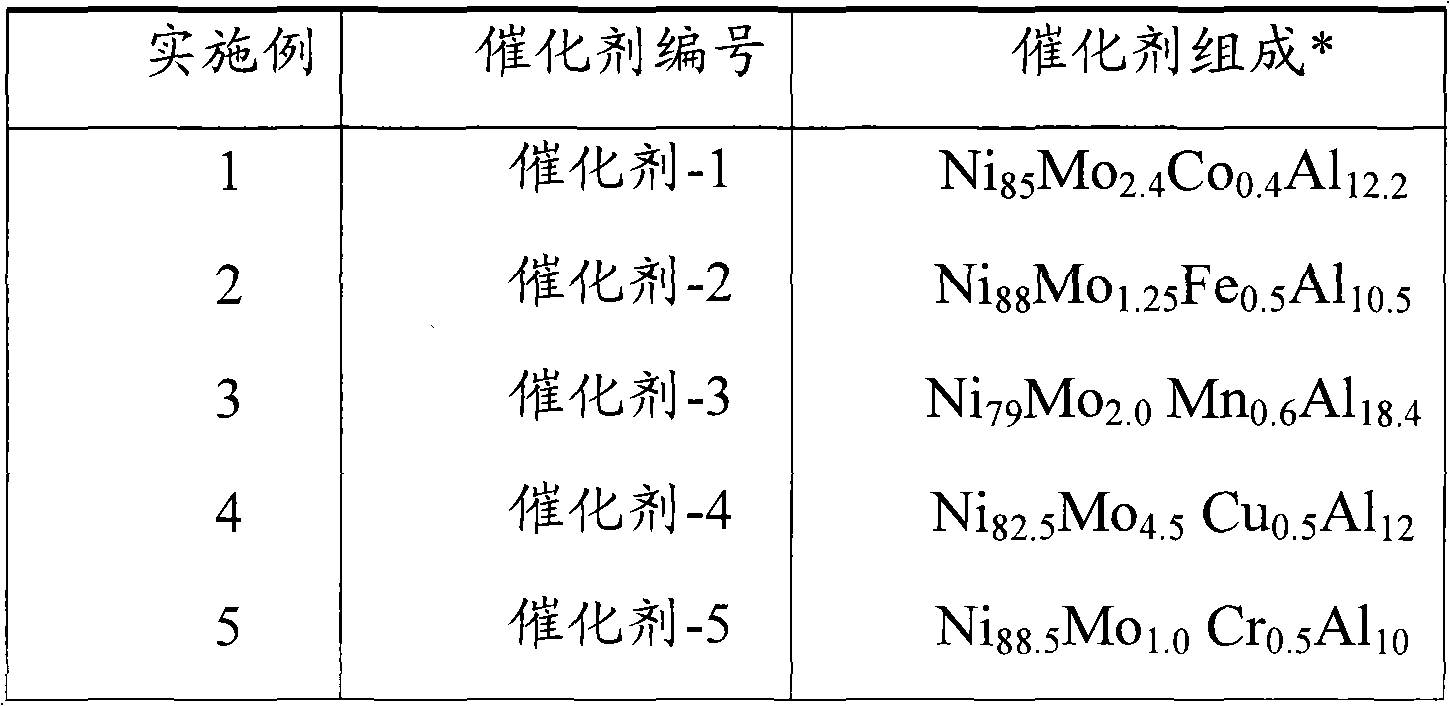
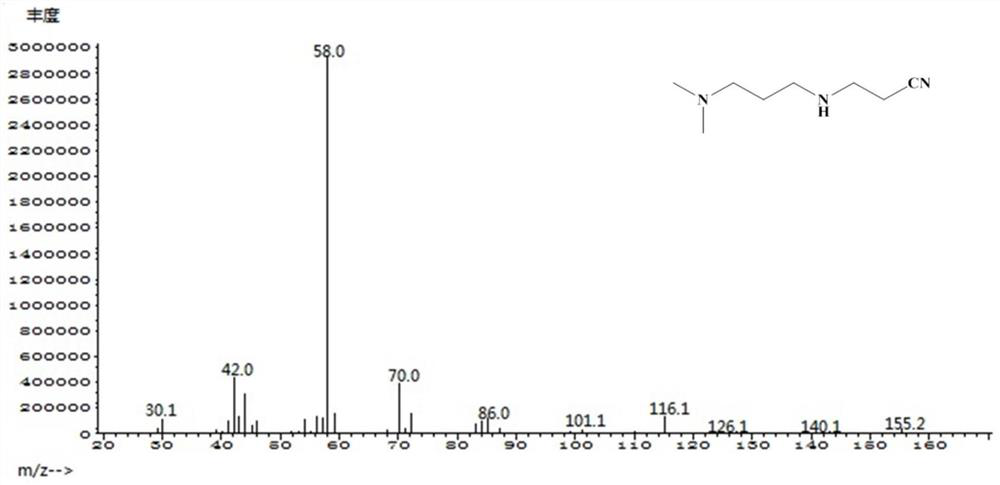
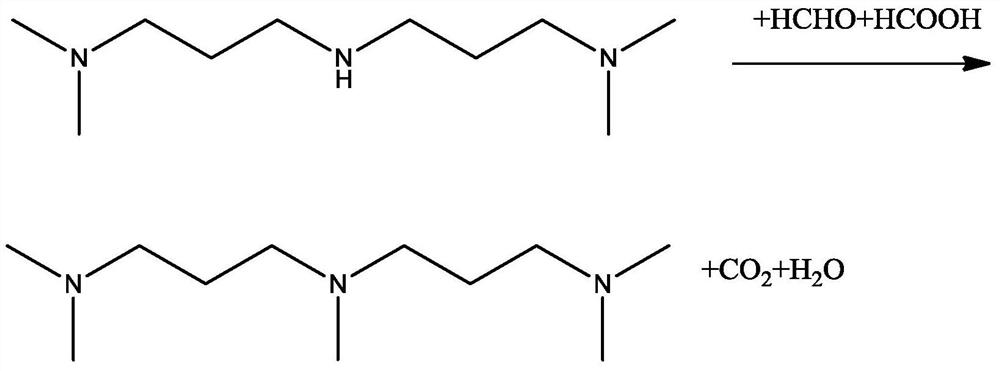
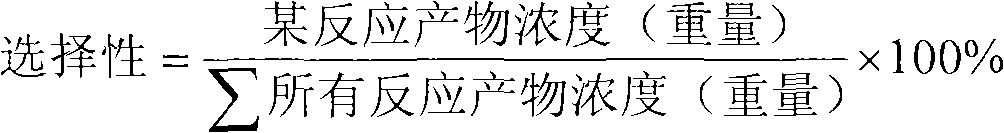Patents
Literature
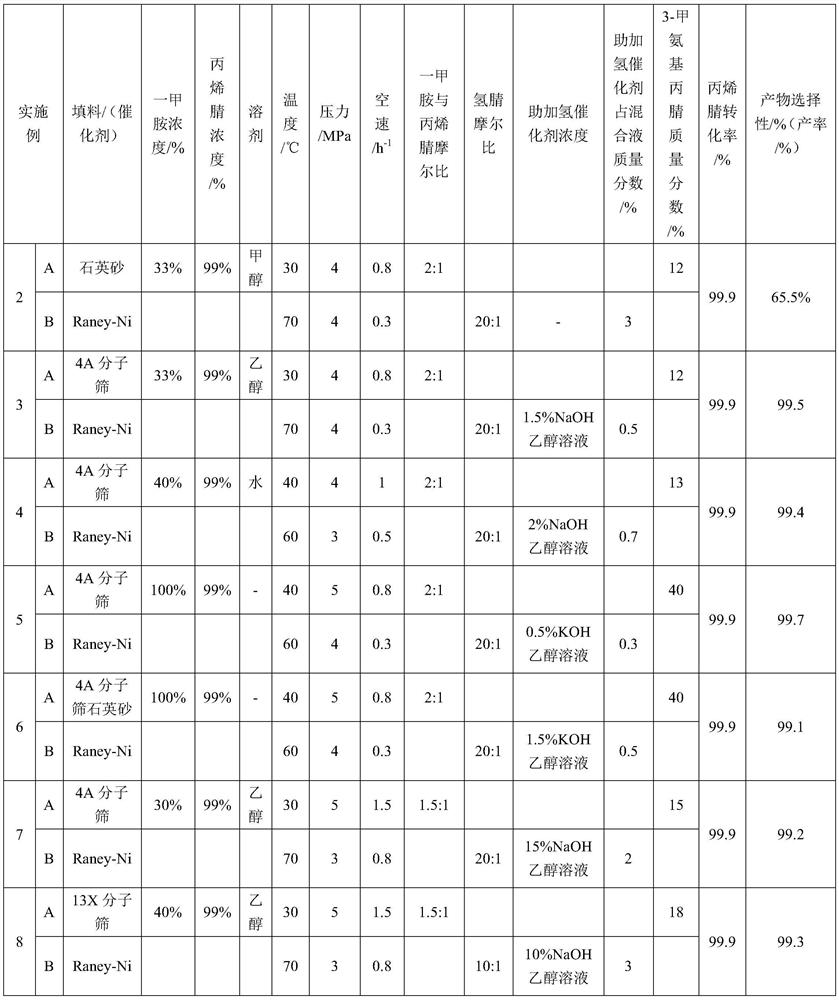
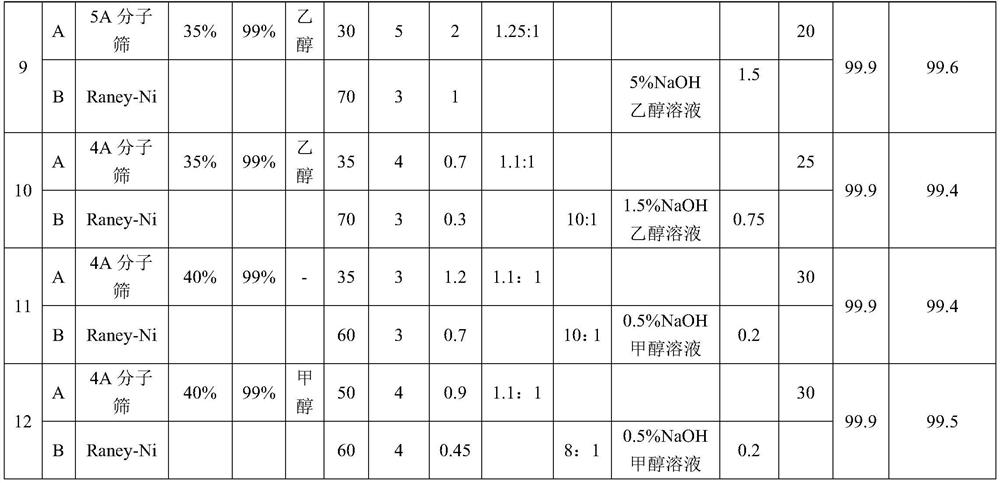
48 results about "Aminopropionitrile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
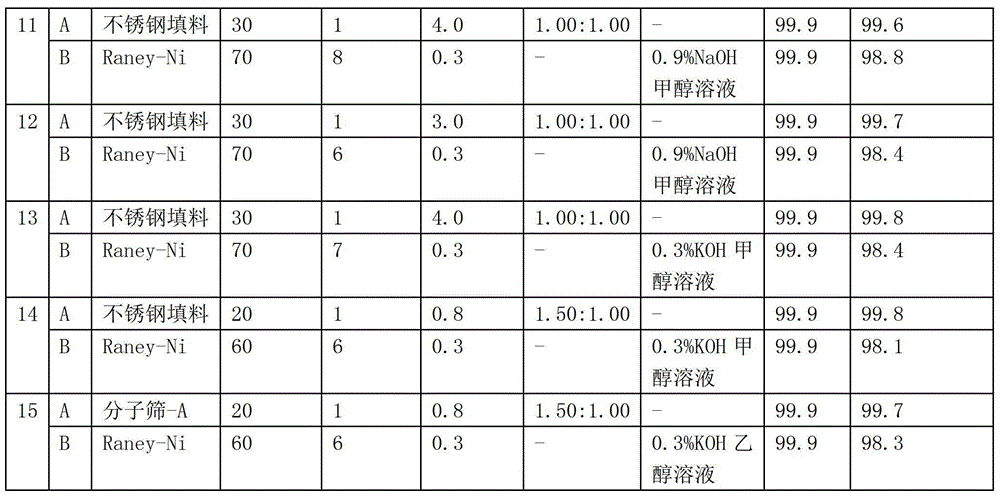
Patent Type
Patent Status
Application Year
Inventor
Aminopropionitrile, also known as β-aminopropionitrile (BAPN), is an organic compound with both amine and nitrile functional groups. It is a colourless liquid. The compound occurs naturally and is of interest in the biomedical community.
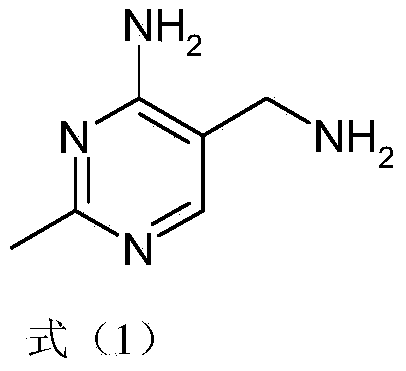
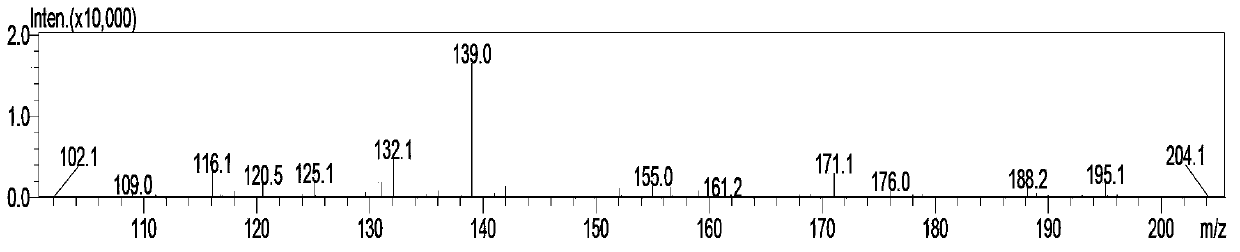
Simple and quick method for synthesizing improved vitamin B1 intermediate 2-methyl-4-amino-5-aminomethylpyrimidine
ActiveCN103435556AReduce concentrationReduce decompositionOrganic chemistryAminopropionitrilePropionitrile
The invention relates to a simple and quick method for synthesizing an improved vitamin B1 intermediate 2-methyl-4-amino-5-aminomethylpyrimidine. The method comprises the following steps: condensing 3-alkyl (aryl) formamido-propionitrile serving as a raw material with acetamidine in the catalytic action of lewis acid; cyclizing with trimethyl orthoformate, and hydrolyzing under an alkaline condition to prepare the vitamin B1 key intermediate 2-methyl-4-amino-5-aminomethylpyrimidine. The four reacting processes are performed in sequence by a one-pot reaction, and the product in each step does not need to be separated and purified. According to the method, highly carcinogenic o-chloroaniline or other small molecular aniline compounds are not used, residues of o-chloroaniline compounds in the vitamin B1 product can be eliminated. Furthermore, the preparation process is short and convenient in flow, small in wastewater amount and high in yield.
Owner:XINFA PHARMA
Simple and convenient preparation method of key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for vitamin B1
The invention relates to a simple and convenient preparation method of a key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for the vitamin B1. According to the method, lewis acid is used for catalyzing addition condensation reaction of acetamidine and 3-formyl amino ethyl cyanide and then catalyzing reaction of the condensation product and triethyl orthoformate to introduce a formyl chiral auxiliary, an imdo group and the formyl chiral auxiliary are subjected to ring formation to form 2- methyl-4-formyl amino-5- amino methyl pyrimidine, and finally basic hydrolysis is performed, so that 2-methyl-4-amino-5-amino methyl pyrimidine is obtained. The reaction procedures are carried out sequentially by adopting the one-pot method, the products in each step require no separation and purification, and the operation is simple and convenient. According to the method, highly toxic o-chloroaniline and other phenylamine compounds are not used, so that residue of o-chloroaniline compounds in the vitamin B1 product can be completely avoided. Meanwhile, the production wastewater is less and the yield is high.
Owner:XINFA PHARMA
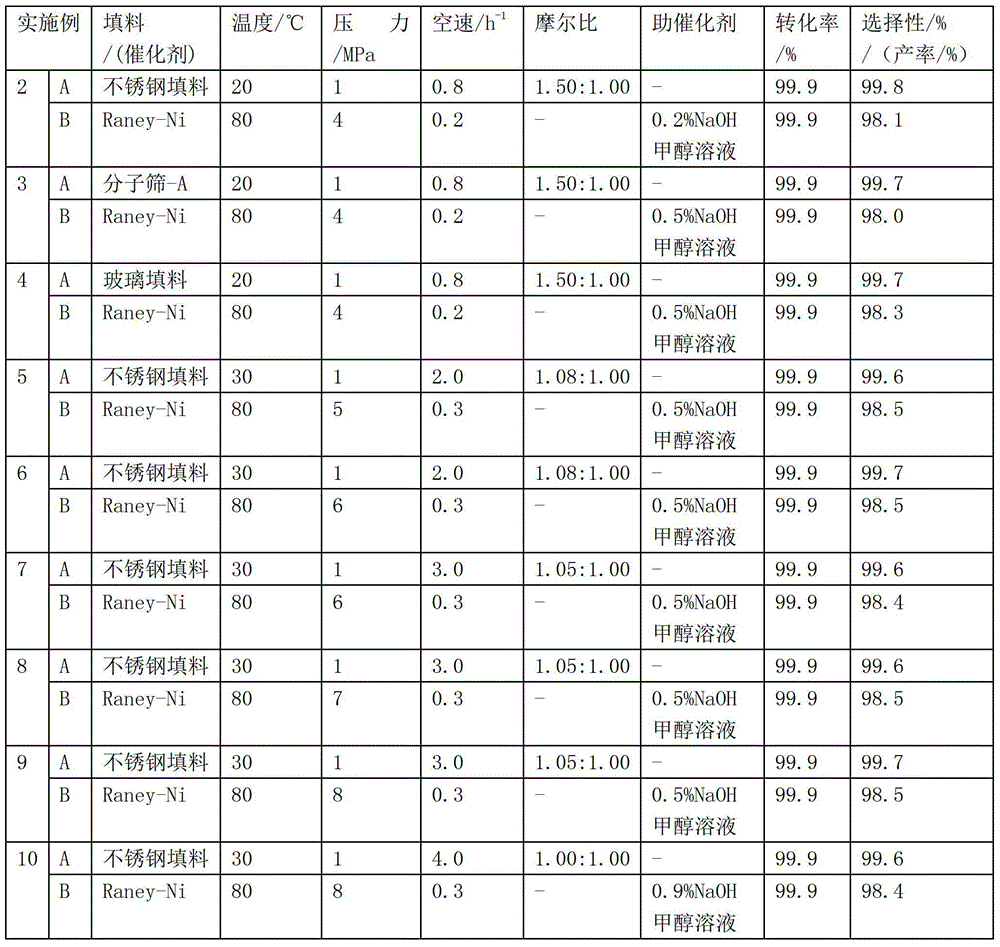
Process for preparing N,N-dimethyl-1,3-propane diamine through continuous method
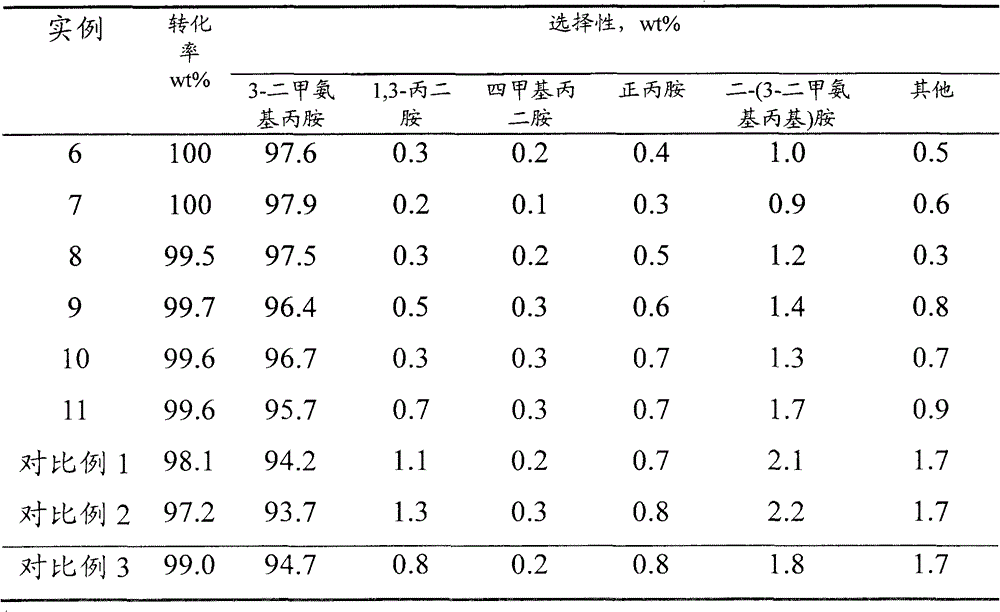
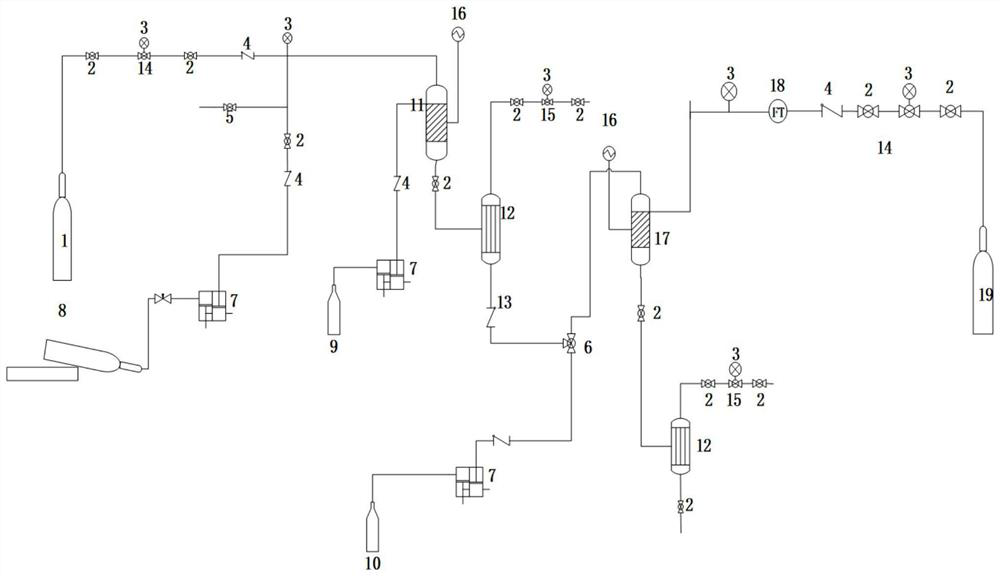
ActiveCN103333073AHigh selectivityImprove conversion rateOrganic compound preparationAmino compound preparationAminopropionitrileAlcohol
The invention discloses a process for preparing N,N-dimethyl-1,3-propane diamine through a continuous method and belongs to the field of organic chemical industry. The process comprises the following steps: by taking dimethylamine and acrylonitrile as raw materials, continuously preparing dimethylamino propionitrile by employing a fixed bed, wherein the molar ratio of dimethylamine to acrylonitrile is (10:1)-(1:1), the reaction temperature is 10-120 DEG C, the air speed is 0.1-10h<-1>, and the conversion rate of the acrylonitrile and the selectivity of the dimethylamino propionitrile are over 99 percent; and allowing the obtained dimethylamino propionitrile intermediate to directly enter a second fixed bed reactor for hydrogenating without any purification treatment, wherein the hydrogenation pressure is 3-10 MPa, a Raney-Ni catalyst is used, an alcoholic solution promoter with 0.1-10 percent of alkali is matched, the air speed is 0.1-4h<-1>, and the yield of N,N-dimethyl-1,3-propane diamine is not lower than 93 percent. The process for preparing N,N-dimethyl-1,3-propane diamine is simple, stable in quality, energy-saving and environment-friendly and is suitable for large-scale production.
Owner:DALIAN UNIV OF TECH
Method for preparing D-calcium pantothenate
ActiveCN101948402AHigh yieldEase of industrial productionOrganic compound preparationCarboxylic acid amides preparationChemical synthesisAminopropionitrile
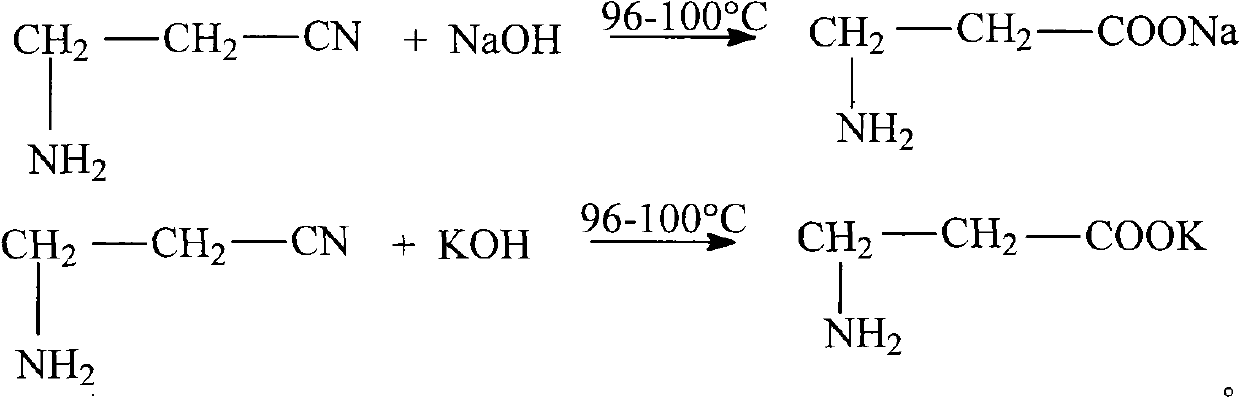
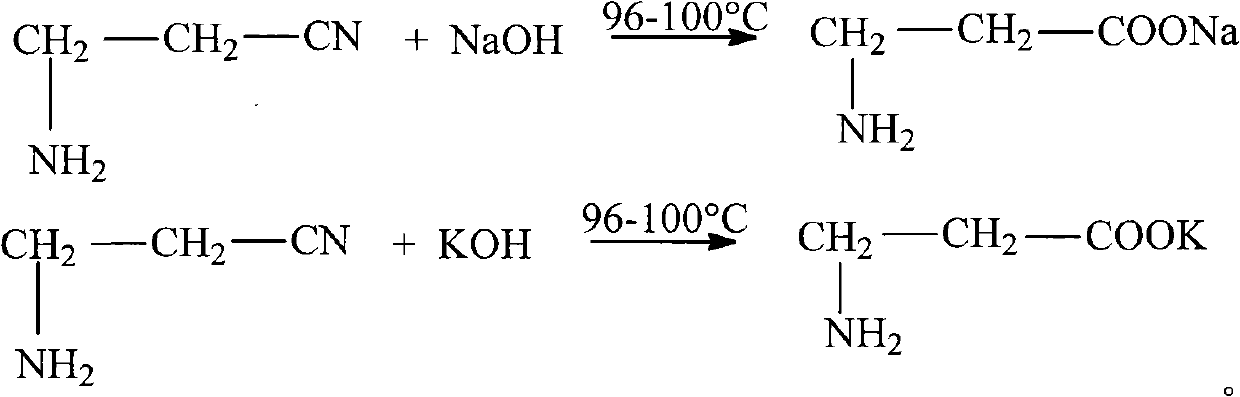
The invention relates to the field of chemical synthesis and discloses a method for preparing D-calcium pantothenate. The method comprises the following steps of: reacting 3-aminopropionitrile with aqueous solution of sodium hydroxide or potassium hydroxide at the temperature of between 96 and 100 DEG C, adding hydrochloric acid to regulate the pH value, and concentrating and crystallizing; dissolving the prepared crystal by using methanol, and filtering to remove salt; and performing ion exchange with 732-type calcium ion exchange resin, carrying out acylation reaction between reaction liquid and D-pantolactone, crystallizing at the temperature of between 15 DEG C below zero and 10 DEG C below zero, and drying to prepare the D-calcium pantothenate, wherein the molar ratio of the 3-aminopropionitrile to the sodium hydroxide and the potassium hydroxide is 1:1.1-1.4, and the molar ratio of the 3-aminopropionitrile to the D-pantolactone 1:1-1.1. The method for preparing the D-calcium pantothenate has the advantages of not using calcium oxide or calcium hydroxide as a calcification agent, avoiding water generation in the reaction process so as to ensure the smooth acylation process, along with simple process, high yield, recycled 732-type calcium ion exchange resin, no generation of three wastes, and contribution to industrial production.
Owner:XINFA PHARMA
Preparation process method of N-methyl-1, 3-propane diamine
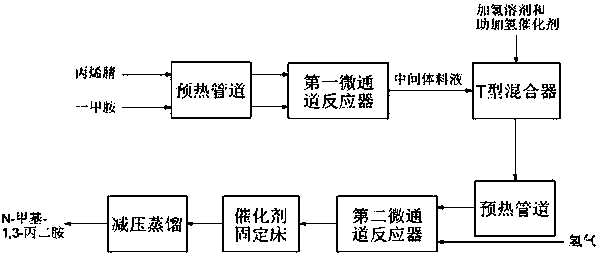
ActiveCN111454159AQuick disperse transferWell mixedCarboxylic acid nitrile preparationOrganic compound preparationAminopropionitrilePtru catalyst
The invention discloses a preparation process method of N-methyl-1, 3-propane diamine, and belongs to the technical field of organic synthesis. According to the method, monomethylamine and acrylonitrile are used as raw materials, an intermediate 3-methylaminopropionitrile is generated through a reaction in a micro-channel reactor, the intermediate is mixed with hydrogen and enters a catalyst fixedbed for a hydrogenation reaction, and finally the product N-methyl-1, 3-propane diamine is obtained. According to the preparation process method of N-methyl-1, 3-propane diamine, the method is simple, continuous production can be achieved, the reaction time is short, the side reaction occurrence probability is low, the product yield reaches 90-95%, and the productivity is improved.
Owner:DALIAN UNIV OF TECH +1
Method for preparing dimethylamino propylamine through hydrogenating dimethylamino propionitrile in presence of nickel
ActiveCN102050742AHigh selectivityLow operating costOrganic compound preparationAmino compound preparationHydrogenAminopropionitrile
The invention relates to a method for preparing dimethylamino propylamine through hydrogenating dimethylamino propionitrile in the presence of nickel. The method comprises the following step: under the reaction condition thatdimethylamino propionitrile is hydrogenated to prepare dimethylamino propylamine in the presence of a catalyst, contacting dimethylamino propionitrile with hydrogen for reaction, wherein the catalyst contains at least one amorphous Ni-Al-Mo-M catalyst; by taking the weight of the catalyst as a reference, the weight ratio of Ni to Al to Mo to M is (5-500):(1-150):(1-150):1; and M is selected from one or more of transition metals in IB group, IIB group, IIIB group, IVB group, VIIB group, VIB group except for Mo and VIII group except for Ni. Compared with the prior art, the invention has the advantage that the selectivity of the dimethylamino propylamine prepared through hydrogenation by dimethylamino propionitrile is obviously increased.
Owner:CHINA PETROLEUM & CHEM CORP +1
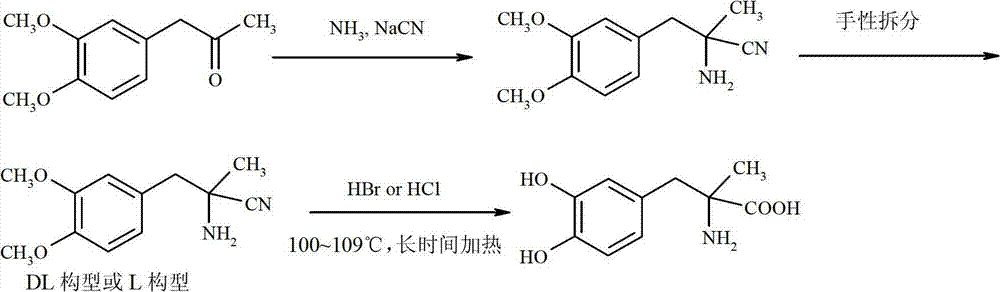
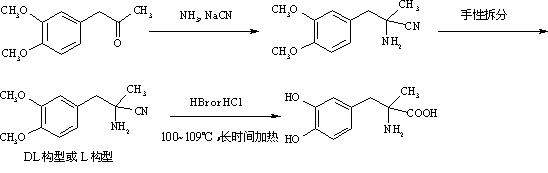
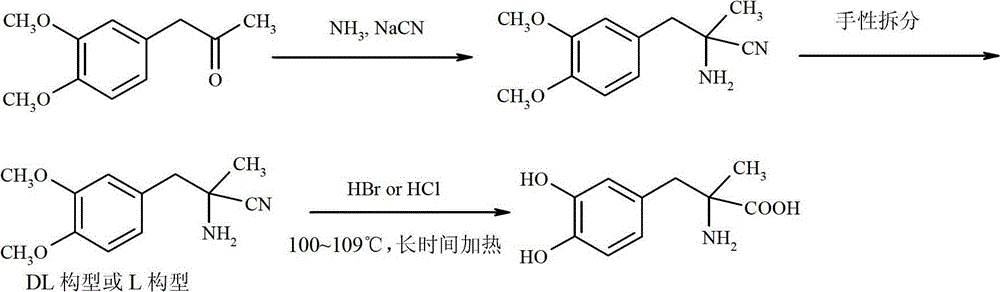
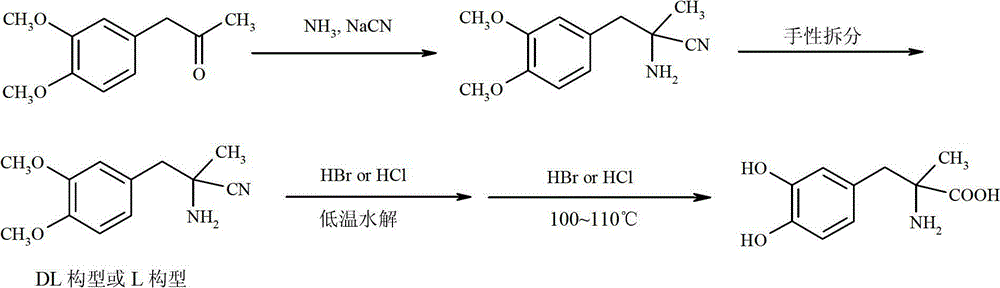
Preparation of methyldopa by hydrolyzing alpha-methyl-(3,4-dimethoxyphenyl)-alpha-aminopropionitrile by two-step hydrolysis method
InactiveCN102786428AEmission reductionReduce energy costsOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisAminopropionitrile
The invention belongs to the technical field of nitrile group hydrolysis and demethylation hydrolysis reaction which is one of the key steps for chemical synthesis of a methyldopa drug or a chiral drug of L-methyldopa [L-alpha-methyl-(3,4-dimethoxyphenyl)-alpha-aminopropionitrile, the same below], that is, a reaction for preparing alpha-methyl-(3,4-dimethoxyphenyl)-alpha-aminopropionitrile or L-alpha-methyl-(3,4-dimethoxyphenyl)-alpha-aminopropionitrile by nitrile group hydrolysis and demethylation hydrolysis. Under an acidic condition, the preparation of methyldopa by nitrile group hydrolysis and demethylation hydrolysis of alpha-methyl-(3,4-dimethoxyphenyl)-alpha-aminopropionitrile (or hydrochloride) requires a long period, a large material using amount, and heating hydrolysis of high-concentration acids (and the long-time heating of the high-concentration acids allows the acids to volatilize, so continuous acid addition is required during hydrolysis); however, the long-time high-concentration acid heating hydrolysis can cause unknown change of certain compound structures of alpha-methyl-(3,4-dimethoxyphenyl)-alpha-aminopropionitrile (or hydrochloride), and thus by-products are increased, and the purity and yield are decreased. Therefore, improvement of the hydrolysis technology is necessary.
Owner:ZHEJIANG UNIV
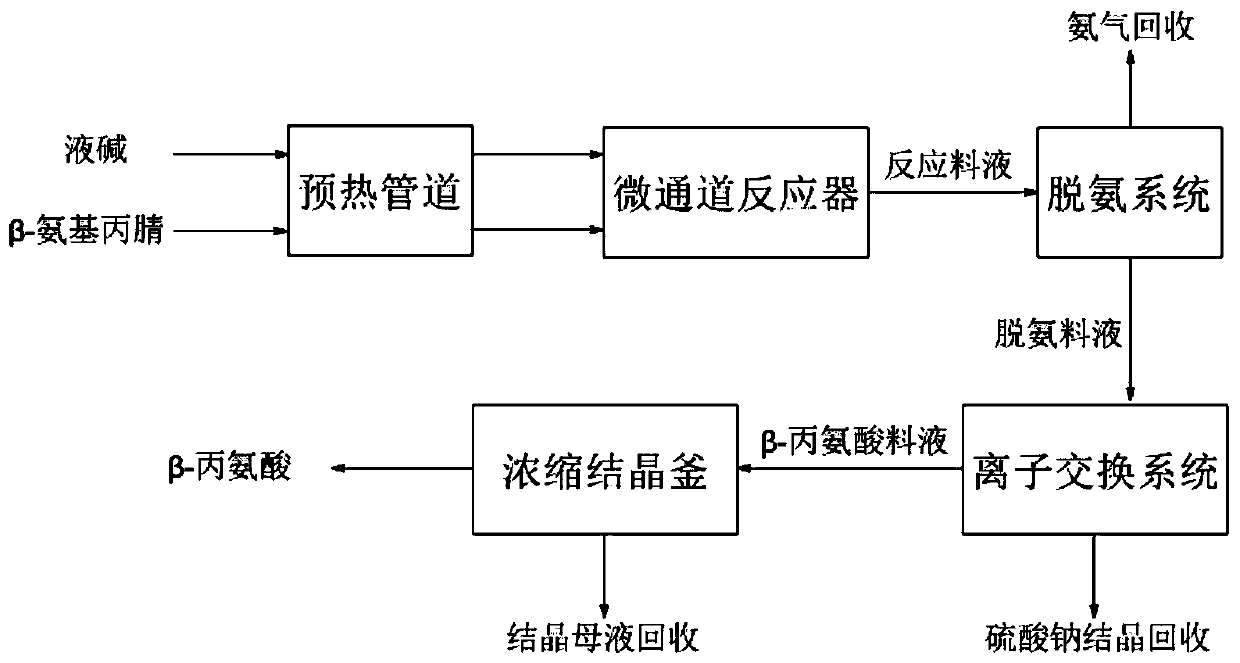
Continuous production process of beta-alanine
InactiveCN111333525AQuick disperse transferInhibit side effectsOrganic compound preparationAmino-carboxyl compound preparationAminopropionitrileIon exchange
The invention belongs to the technical field of beta-alanine, and relates to a continuous production process of beta-alanine. The method comprises the following steps: carrying out instantaneous hydrolysis reaction on the raw material beta-aminopropionitrile and liquid caustic soda through a micro-channel reactor under the conditions of high temperature and high pressure to obtain beta-sodium alanine-containing reaction feed liquid, then removing ammonia gas through a deamination system, exchanging sodium ions through an ion exchange system, and subjecting the exchanged beta-alanine aqueous solution to concentration crystallization to obtain beta-alanine, thus realizing continuous production from feeding to crystallization. According to the method, the reaction time is short, the side reaction occurrence probability is low, the product purity reaches 99.5% or above, and the yield reaches 95% or above; the whole system realizes automatic continuous production, is high in resource utilization rate, saves energy and reduces consumption.
Owner:DALIAN WONDERSUN BIOCHEM TECH
Cockroach pesticide
InactiveCN105494346AMass killing effectEfficient killingBiocideAnimal repellantsBenzoic acidAminopropionitrile
The invention relates to a pesticide, and specifically relates to a cockroach pesticide. The cockroach pesticide is prepared from the following raw materials in parts by weight: 25 to 30 parts of 3-[(2-chlorophenyl)amino]propionitrile, 15 to 18 parts of 1-methyl-4-(5H-dibenzo[a,d]cycloheptatriene-5-ylidene)piperidine hydrochloride, 25 to 28 parts of tranylcypromine hydrochloride, 30 to 32 parts of 4-acetamido-2-ethyoxylmethyl benzoate, and 12 to 18 parts of 1,4-butanediol monomethyl ether. The chemical components cooperate with each other to generate a great killing performance and thus the cockroaches can be killed effectively.
Owner:张媛媛
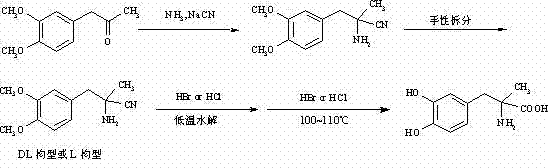
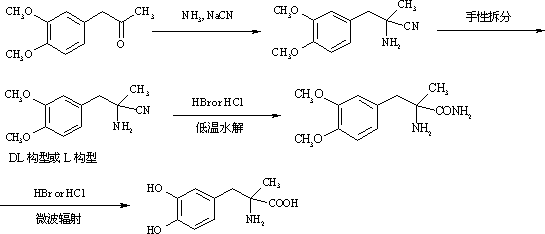
Method for preparing methyldopa from alpha-methyl-(3,4-dimethoxy phenyl)-alpha-aminopropionitrile by microwave hydrolysis method
ActiveCN102702004AEmission reductionHigh purityOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisAminopropionitrile
The invention belongs to the technical field of one critical step in microwave nitrile hydrolysis and demethylation hydrolysis of chemical synthesis medicine methyldopa [alpha-methyl-(3,4-dyhydroxy phenyl)-alpha-aminopropionic acid, similarly hereinafter] or synthetic chiral medicine L-methyldopa [L-alpha-methyl-(3,4-dyhydroxy phenyl)-alpha-aminopropionic acid, similarly hereinafter], i.e. the chemical reaction for preparing methyldopa or L-methyldopa from alpha-methyl-(3,4-dimethoxy phenyl)-alpha-aminopropionitrile or L-alpha-methyl-(3,4-dimethoxy phenyl)-alpha-aminopropionitrile through microwave nitrile hydrolysis and demethylation hydrolysis. The microwave hydrolysis uses low-concentration hydrochloric acid or hydrobromic acid, so the energy sources and the economic cost are reduced, the acid wastewater discharge is reduced, by-products are reduced, and the purity and the yield of products are improved.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
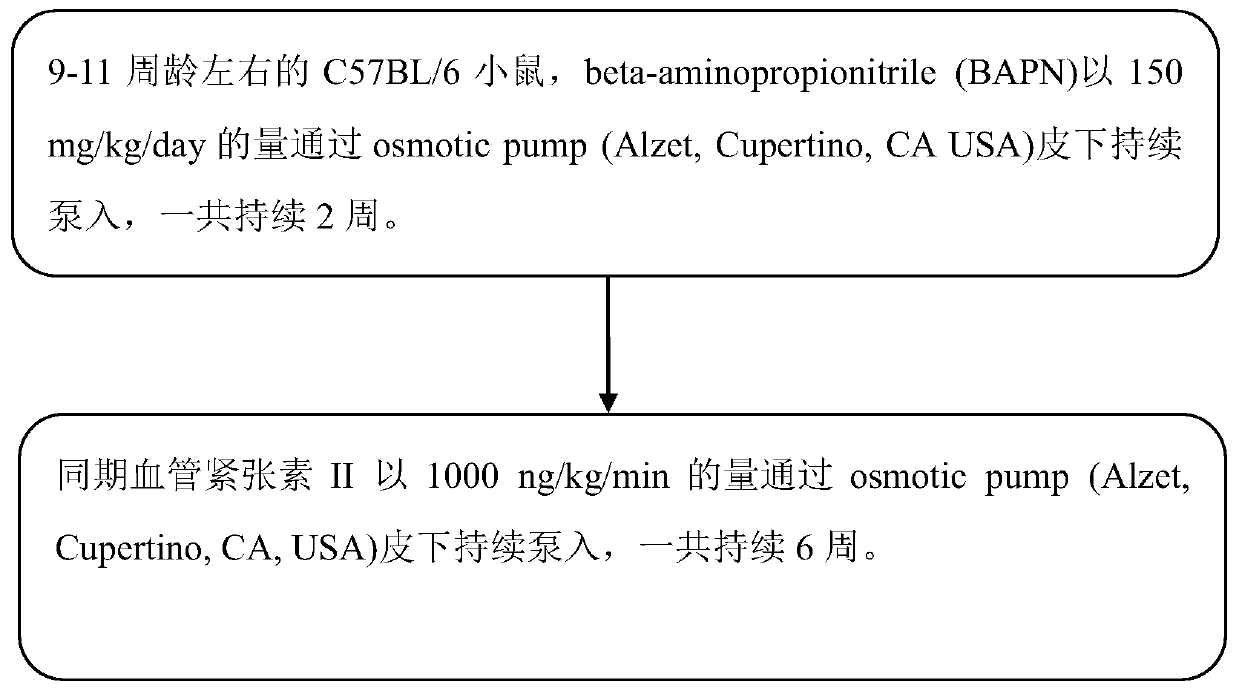
Thoracoabdominal aortic aneurysm mouse model preparation method
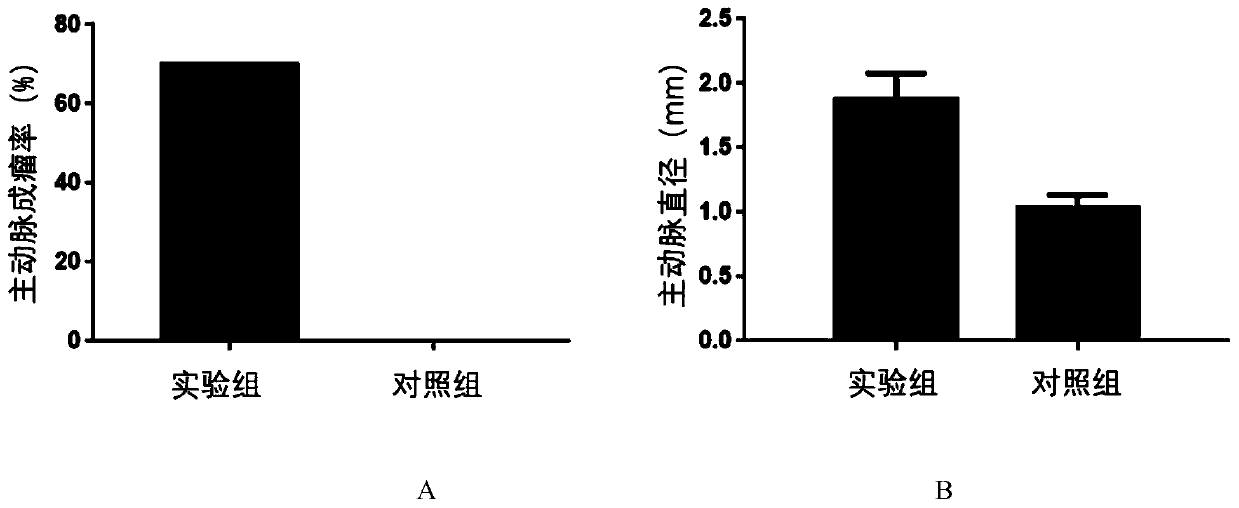
InactiveCN110692599AConform to the evolution processSignificant technological progressAnimal husbandryThoracoabdominal Aortic AneurysmsAminopropionitrile
The invention provides a thoracoabdominal aortic aneurysm mouse model preparation method. The method includes: selecting a C57BL / 6 mouse at the age of 9-11 weeks, and subcutaneously and continuously pumping BAPN (beta-aminopropionitrile) by 150mg / kg / day through an animal trace dosage osmotic pump for two weeks; subcutaneously and continuously pumping angiotensin II by 1000ng / kg / min through an animal trace dosage osmotic pump for six weeks to obtain a thoracoabdominal aortic aneurysm mouse model. By a beta-aminopropionitrile and angiotensin II combined method for inducing formation of thoracoabdominal aortic aneurysm of the C57BL / 6 mouse, the established model better accords with a physiopathologic mechanism of thoracoabdominal aortic aneurysm formation, and further study on an aortic aneurysm pathological mechanism is benefited.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Process for continuously preparing N, N-dimethyl-1, 3-propane diamine by using micro-mixing and fixed bed reactors
PendingCN113200870AReduce spoilageReduced chance of spoilageCarboxylic acid nitrile preparationOrganic compound preparationAminopropionitrileAcrylonitrile
The invention discloses a process for continuously preparing N, N-dimethyl-1, 3-propane diamine by using micro-mixing and fixed bed reactors, and belongs to the technical field of organic chemical industry. A micro-mixing reactor and a fixed bed reactor are connected in series, and dimethylamine and acrylonitrile are used as raw materials to continuously prepare N, N-dimethyl-1, 3-propane diamine. By adopting the micro-mixing reactor, mass transfer and heat transfer of the reaction of dimethylamine and acrylonitrile are greatly improved, the reaction time of the first step is shortened to 0.3-4 min, and the probability of deterioration of the intermediate dimethylaminopropionitrile is reduced. Through the micro-mixing reactor, the gas-liquid raw materials in the second step are mixed more uniformly, the hydrogenation efficiency is improved, the yield of the prepared N, N-dimethyl-1, 3-propane diamine is greater than or equal to 98%, and the acrylonitrile conversion rate is greater than or equal to 99%. The process method can easily realize continuous production, low in cost, green, environment-friendly and suitable for industrial production.
Owner:DALIAN UNIV OF TECH
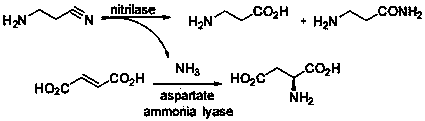
Method for preparing beta-alanine through enzymatically catalyzing hydrolysis of high-concentration beta-aminopropionitrile
The invention relates to a method for preparing beta-alanine through enzymatically catalyzing hydrolysis of high-concentration beta-aminopropionitrile. More specifically, the invention provides a hydrolyzing high-concentration beta-aminopropionitrile by utilizing nitrilase and genetically engineered bacteria thereof or combining together with aspartic acid lyase and genetically engineered bacteria thereof, and the beta-alanine is widely applied to synthesis of pantothenic acid, calcium pantothenate, carnosine, Pamidronate, balsalazine and other medical or feed and food additives. The method has the significant characteristics of being high in substrate concentration, mild in reaction conditions, pollution-free, simple in process route, and the like.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Preparation method of weather-resistant advertising car sticker for outdoor use
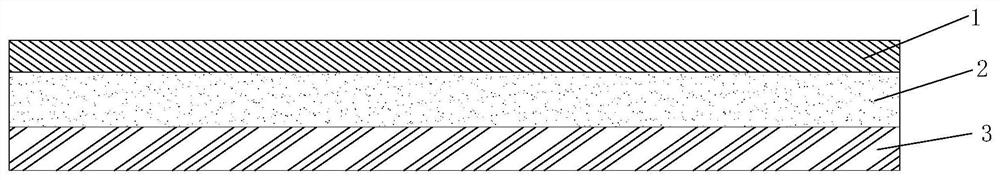
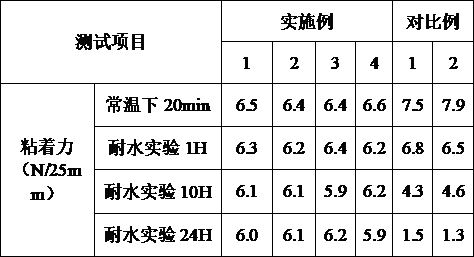
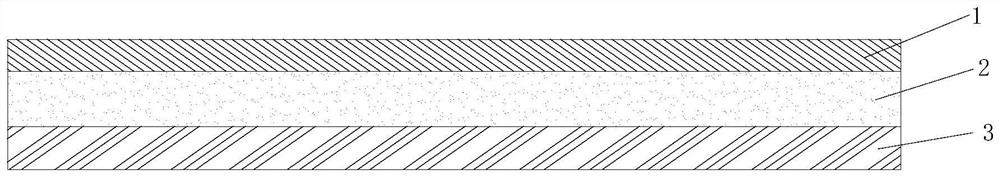
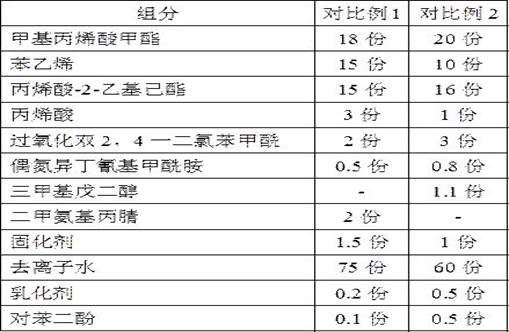
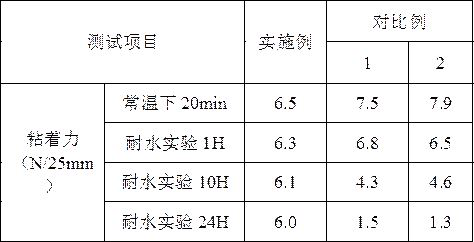
ActiveCN112251155BExtended service lifeImprove waterproof performanceNon-macromolecular adhesive additivesEster polymer adhesivesMeth-Aminopropionitrile
The invention discloses a method for preparing an outdoor weather-resistant advertising car sticker, which comprises a base material layer, an adhesive layer and a release layer, one side of the base material layer is coated on the adhesive layer, and the adhesive layer The side opposite to the substrate layer is provided with a release layer, and the adhesive layer is obtained by coating and drying the glue solution. The glue solution is obtained by the following steps: S1, adding 15 to 25 parts of methyl methacrylate, Mix 15~25 parts of styrene, 15~20 parts of 2-ethylhexyl acrylate, 1~1.5 parts of trimethylpentanediol, 1~5 parts of dimethylaminopropionitrile and 50~75 parts of deionized water , heat up to 75~80°C, add dropwise 0.5~1.5 parts of azoisobutylcyanoformamide and 1~10 parts of acrylic acid to the mixed system, and react for 3~4 hours; S2, cool to room temperature, add bis peroxide 2 , 1-5 parts of 4-dichlorobenzoyl, 1-1.5 parts of curing agent, 0.2-1 part of emulsifier and 0.1-1 part of hydroquinone, mix evenly to obtain glue; S3, evenly coat the glue On the substrate layer, dry to obtain the adhesive layer. The invention improves the waterproof performance, the adhesive force is basically unchanged, and the bonding is stable.
Owner:ZHEJIANG FULAI NEW MATERIAL CO LTD
Process for preparing N-methyl-1,3-propane diamine through continuous catalytic reaction by using two fixed bed reactors
ActiveCN112961061AEasy to achieve serial productionLow costCarboxylic acid nitrile preparationOrganic compound preparationAminopropionitrileAcrylonitrile
The invention discloses a process for preparing N-methyl-1,3-propane diamine through continuous catalytic reaction by using two fixed bed reactors, belonging to the technical field of organic chemical industry. According to the invention, monomethylamine and acrylonitrile are used as raw materials, a 3-methylaminopropionitrile intermediate is generated through continuous reaction in a first fixed bed reactor, and then the intermediate continuously enters a second fixed bed hydrogenation reactor for catalytic hydrogenation, so N-methyl-1,3-propane diamine is generated, and the yield is larger than or equal to 99%. The process disclosed by the invention is continuous in production, simple in process, less in three wastes, reduced in energy consumption, friendly to environment, low in cost and easy to industrialize.
Owner:DALIAN UNIV OF TECH
Process for continuously preparing N-hydroxyethyl-1, 3-propane diamine by using micro-mixing and fixed bed reactor
PendingCN113149850AWell mixedEnhance heat and mass transferCarboxylic acid nitrile preparationOrganic compound preparationAminopropionitrilePtru catalyst
The invention discloses a process for continuously preparing N-hydroxyethyl-1, 3-propane diamine by using micro-mixing and a fixed bed reactor, and belongs to the technical field of organic chemical industry. The process comprises the following steps: taking ethanolamine and acrylonitrile as raw materials, firstly, continuously reacting through a first fixed bed reactor to generate an N-hydroxyethyl-3-aminopropionitrile intermediate, then, carrying out liquid-liquid mixing on the N-hydroxyethyl-3-aminopropionitrile intermediate and a cocatalyst in a first micro-mixing reactor, and then, carrying out gas-liquid mixing on the N-hydroxyethyl-3-aminopropionitrile intermediate and hydrogen in a second micro-mixing reactor together; finally, the intermediate reaction liquid continuously enters a second fixed bed hydrogenation reactor for catalytic hydrogenation, N-hydroxyethyl-1, 3-propane diamine is generated, and the yield is larger than or equal to 98%. The process is continuous in production, simple, less in three wastes, energy-saving, environment-friendly, low in cost and easy to industrialize.
Owner:DALIAN UNIV OF TECH
Method for preparing 3-aminopropionitrile from waste liquid containing 3,3-iminodipropionitrile
InactiveCN112679382AHigh yieldAchieve reuseOrganic compound preparationCarboxylic acid nitrile purification/separationAminopropionitrileDistillation
The invention provides a method for preparing 3-aminopropionitrile from a waste liquid containing 3,3-iminodipropionitrile, wherein the method comprises the following steps: respectively introducing the waste liquid containing 3,3-iminodipropionitrile and an ammonia water solution into a micro-channel reactor to react, to obtain a reaction feed liquid; and carrying out deamination and distillation on the reaction feed liquid to obtain 3-aminopropionitrile. Firstly, the treatment method for preparing the 3-aminopropionitrile from the 3,3-iminodipropionitrile in the 3,3-iminodipropionitrile waste liquid can effectively realize reutilization of resources, and the yield of the 3-aminopropionitrile is relatively high; and secondly, the micro-channel reactor is adopted to strengthen mass and heat transfer in the reaction process, improve the reaction efficiency and realize continuous synthesis of the process, so that the high-temperature residence time can be shortened, the whole process is easy to control, the reutilization of beta-aminopropionitrile byproducts is improved favourably, and the consumption is reduced.
Owner:HANGZHOU XINFU TECH CO LTD
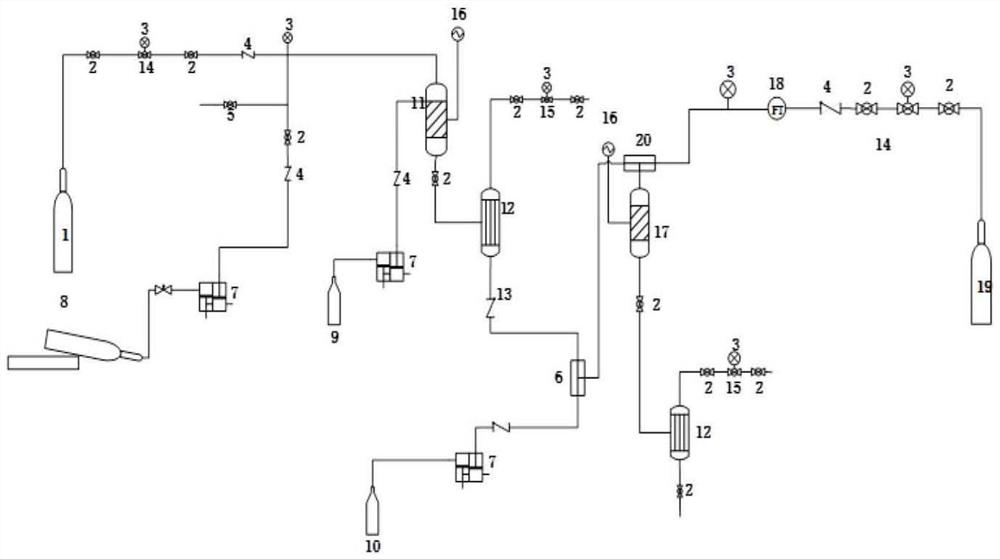
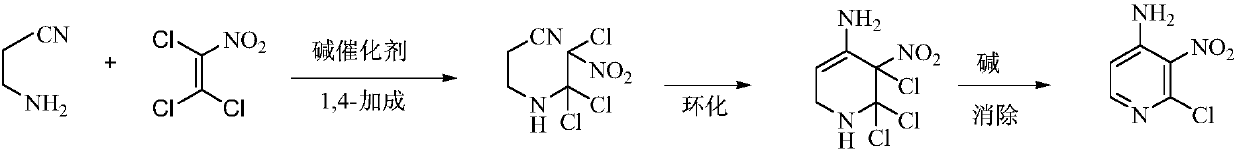
Environmental protection preparation method of 4-amino-2-chloro-3-nitropyridine
The invention relates to an environmental protection preparation method of 4-amino-2-chloro-3-nitropyridine. The method uses 3-amino propionitrile and trichloronitroethylene for 1,4-Addition and cyclization reaction to obtain 4-amino--2, 2, 3-trichloro-3-nitro-1, 2, 3, 6-tetrahydropyridine, and eliminates hydrogen chloride with an acid binding agent to prepare the 4-amino-2-chloro-3-nitropyridine.The method has the advantages of good reaction selectivity, good target product yield, high purity, short process flow, safe and simple operation, mild conditions, no waste acid and wastewater, environmental protection and low cost, and is carried out by a one-pot method.
Owner:XINFA PHARMA
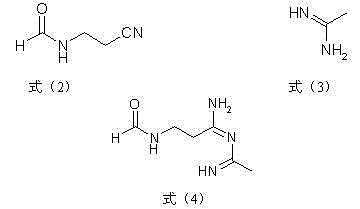
Preparation method and application of alpha-sodio formyl-beta-formylaminopropionitrile
ActiveCN112778158AHigh synthetic yieldReduce consumptionCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisSodium methoxide
The invention provides a preparation method and application of alpha-sodio formyl-beta-formylaminopropionitrile, and relates to the technical field of chemical synthesis. The preparation method of the alpha-sodio formyl-beta-formylaminopropionitrile comprises the following steps of: firstly, mixing beta-aminopropionitrile with methyl formate, and carrying out formylation reaction to obtain a pre-acylation solution; then sequentially adding sodium methoxide and isopropanol into the pre-acylation solution to be mixed, and conducting a synthetic reaction to obtain a solution containing alpha-sodium formyl-beta-formylaminopropionitrile; and finally, sequentially filtering and drying to obtain the alpha-sodio formyl-beta-formylaminopropionitrile. According to the preparation method, the sodio synthesis yield is effectively improved, and detection shows that the sodio synthesis yield can reach about 90% and is far higher than the existing sodio synthesis yield which is about 64%; and meanwhile, the preparation method also has the effects of reducing the consumption of methyl formate and reducing the generation of sodio mother liquor high-boiling residues.
Owner:江苏兄弟维生素有限公司
Preparation method and application of alpha-(o-chloroaniline) methylene-beta-formylaminopropionitrile
PendingCN114230488AAvoid throwing wasteAvoid environmental problemsCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisAminopropionitrile
The invention provides a preparation method and application of alpha-(o-chloroaniline)-based methenyl-beta-formylaminopropionitrile, and relates to the technical field of chemical synthesis.The preparation method comprises the steps that an aqueous solution of alpha-sodium formyl-beta-formylaminopropionitrile and an acid solution of o-chloroaniline are subjected to a reaction, and alpha-(o-chloroaniline)-based methenyl-beta-formylaminopropionitrile and o-chloroaniline are subjected to a reaction to obtain alpha-(o-chloroaniline)-based methenyl-beta-formylaminopropionitrile; the alpha-(o-chloroaniline)-based methine-beta-formylamino propionitrile is obtained. The method solves the technical problems that solid alpha-sodium formyl-beta-formylaminopropionitrile is taken as a raw material in the traditional synthesis process, so that the operation environment is polluted by turnover throwing, the labor intensity is high, the operation period is long, the operation requirement is complex and the like; the technical effects of small operation environment pollution, simple operation, low labor intensity and short operation period are achieved.
Owner:江苏兄弟维生素有限公司
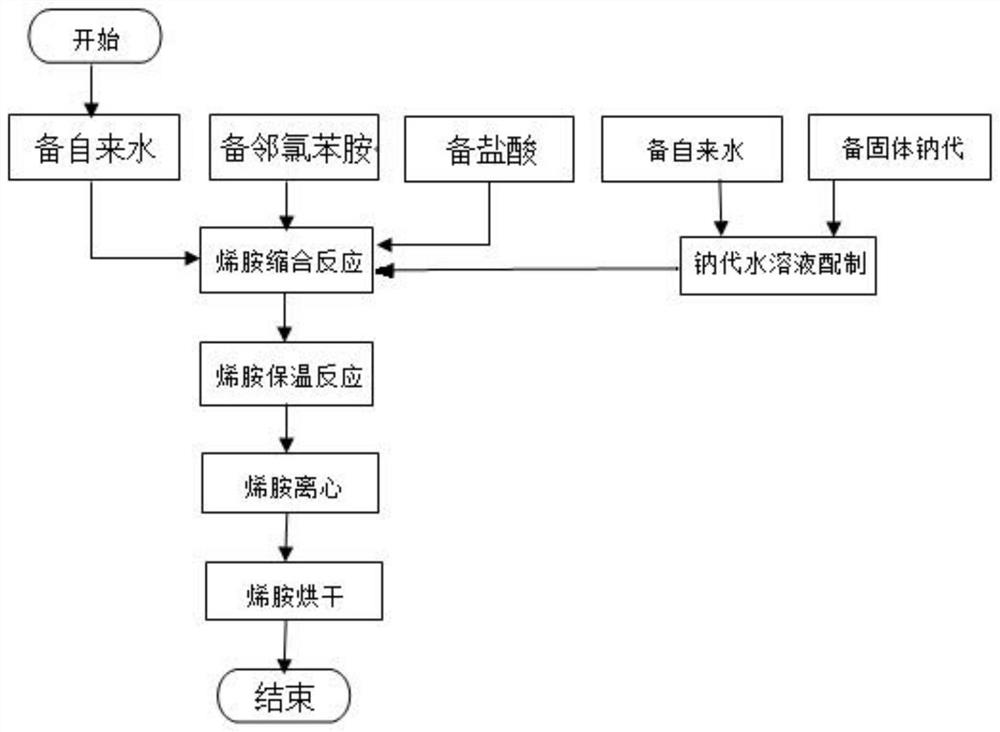
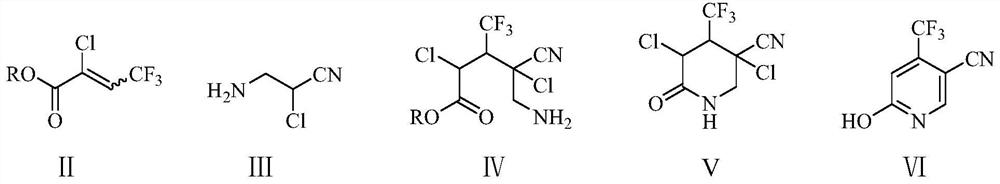
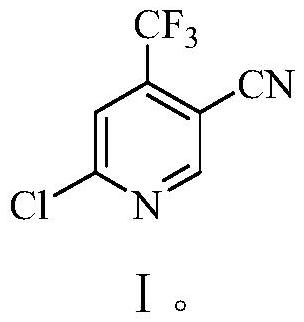
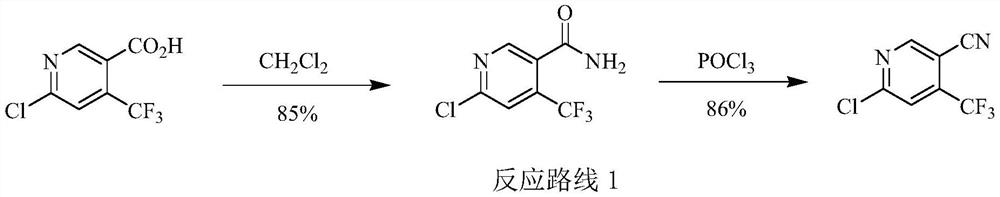
Preparation method of 6-chloro-4-trifluoromethyl-3-cyanopyridine
ActiveCN113912535AHigh yieldHigh purityOrganic chemistryBulk chemical productionMethacrylateAminopropionitrile
The invention provides a preparation method of 6-chloro-4-trifluoromethyl-3-cyanopyridine, which comprises the following steps: by taking 2-chloro-3-trifluoromethyl acrylate (II) and 2-chloro-3-aminopropionitrile (III) as raw materials, carrying out addition reaction to obtain 2,4-dichloro-3-trifluoromethyl-4-cyano-5-amino valerate (IV), carrying out molecular lactamization reaction to obtain 3,5-dichloro-4-trifluoromethyl-5-cyano piperidine-2-one (V), then removing hydrogen chloride to obtain 6-hydroxy-4-trifluoromethyl-3-cyanopyridine (VI), and then carrying out chlorination reaction on VI and a chlorination reagent to obtain 6-chloro-4-trifluoromethyl-3-cyanopyridine (I). The raw materials used in the invention are cheap and easily available; the preparation and operation method is simple, small in wastewater amount, safe, environment-friendly and low in cost; the method has the advantages of high reaction selectivity, few byproducts and high target product yield and purity, and is suitable for green industrial production.
Owner:XINFA PHARMA
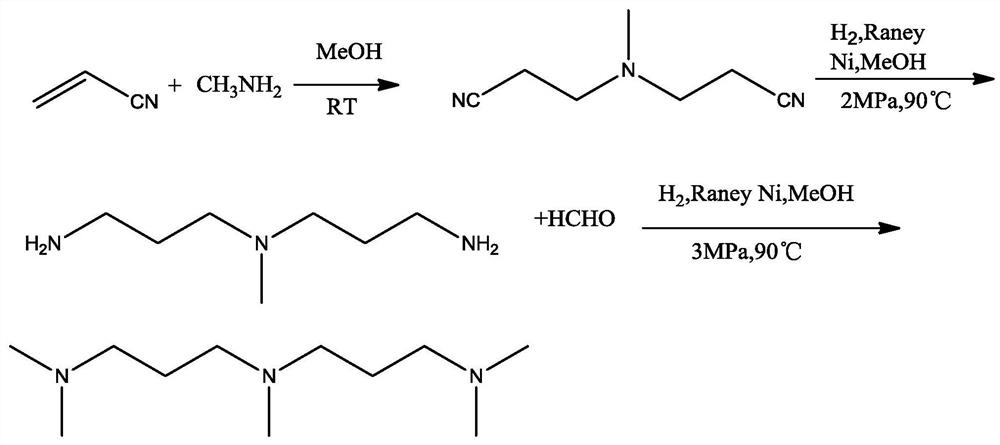
A kind of method for preparing pentamethyldipropylene triamine
ActiveCN110437102BHigh yieldHigh cost advantageAmino preparation from aminesCarboxylic acid nitrile preparationAminopropionitrilePtru catalyst
Owner:WANHUA CHEM GRP CO LTD +1
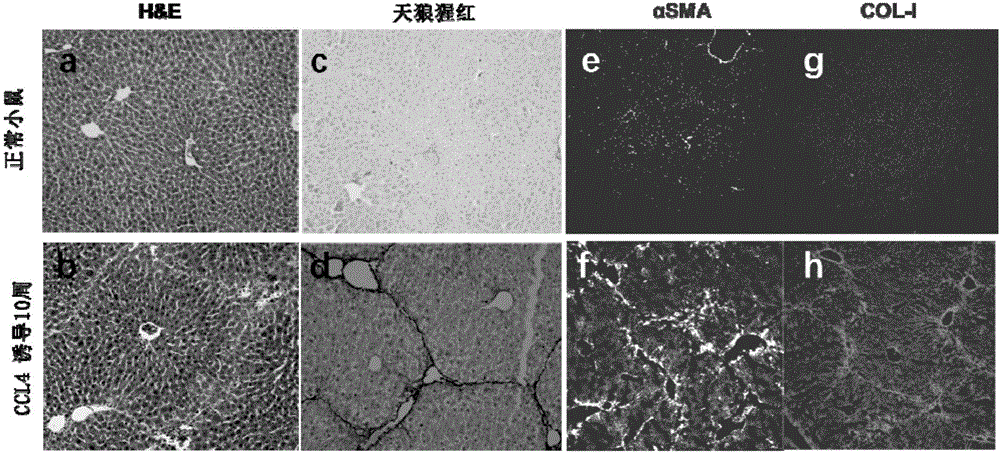
Use of aminopropionitrile in preparation of medicine
InactiveCN106492218AGood water solubilityPromote absorptionDigestive systemPharmaceutical delivery mechanismFiberDesmin
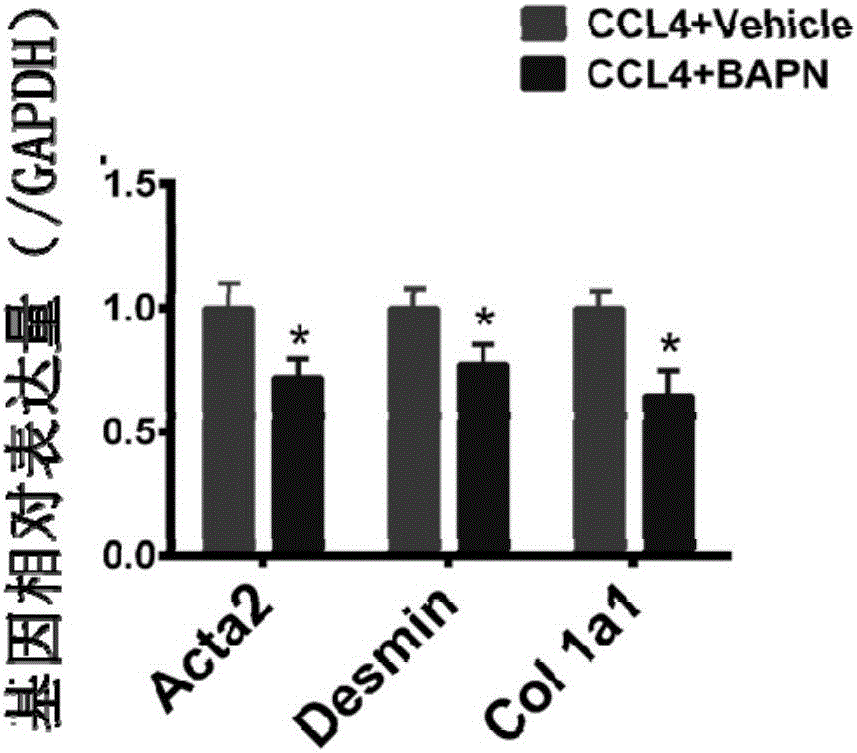
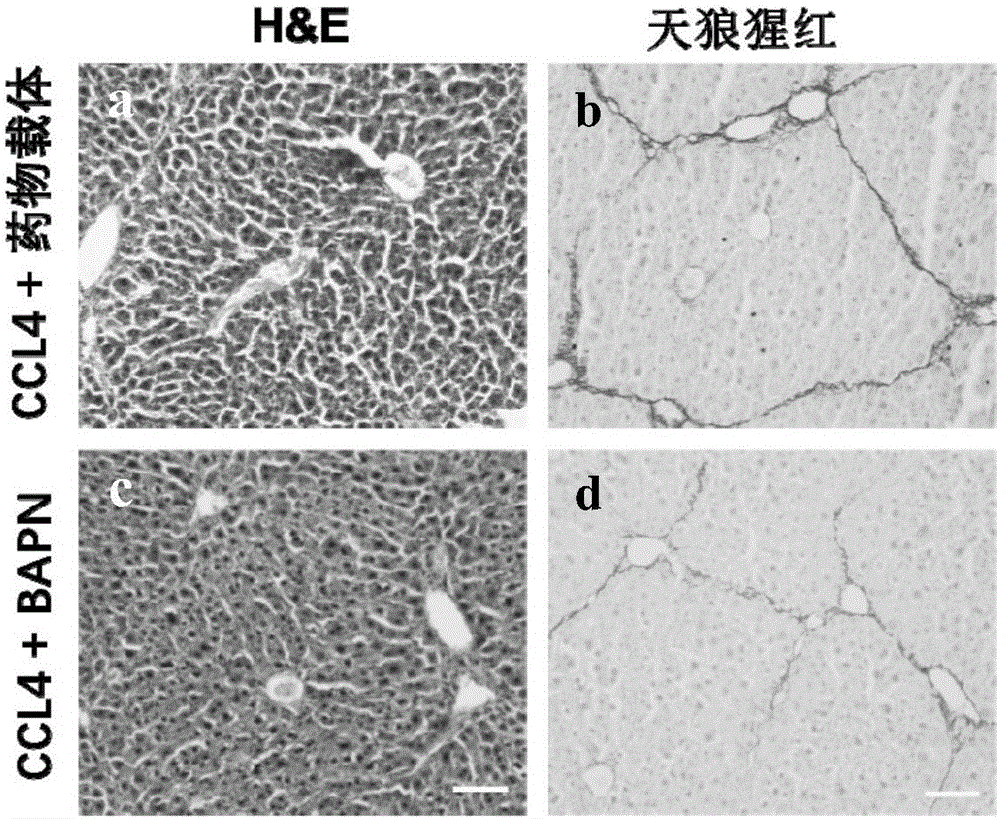
A use of aminopropionitrile in preparation of a medicine is provided. The medicine is used for treating late-stage hepatic fibrosis and cirrhosis. When compared with a contrast group free of aminopropionitrile, the medicine containing the aminopropionitrile can effectively reduce expression of fibrosis marker genes which are col lal, Acta2 and Desmin of mice with F3-stage hepatic fibrosis (based on METAVIR staging criteria), and can allow mouse liver tissue structures to be complete, collagenous fiber deposition to be reduced, collagenous fibers to be thinner, a collagen structure to be loose and dispersed, and activated astrocytes to be reduced, and therefore it is proved that the aminopropionitrile has obvious improving effects on hepatic fibrosis and cirrhosis.
Owner:TSINGHUA UNIV
Simple and convenient preparation method of key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for vitamin B1
The invention relates to a simple and convenient preparation method of a key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for the vitamin B1. According to the method, lewis acid is used for catalyzing addition condensation reaction of acetamidine and 3-formyl amino ethyl cyanide and then catalyzing reaction of the condensation product and triethyl orthoformate to introduce a formyl chiral auxiliary, an imdo group and the formyl chiral auxiliary are subjected to ring formation to form 2- methyl-4-formyl amino-5- amino methyl pyrimidine, and finally basic hydrolysis is performed, so that 2-methyl-4-amino-5-amino methyl pyrimidine is obtained. The reaction procedures are carried out sequentially by adopting the one-pot method, the products in each step require no separation and purification, and the operation is simple and convenient. According to the method, highly toxic o-chloroaniline and other phenylamine compounds are not used, so that residue of o-chloroaniline compounds in the vitamin B1 product can be completely avoided. Meanwhile, the production wastewater is less and the yield is high.
Owner:XINFA PHARMA
Method for preparing dimethylamino propylamine through hydrogenating dimethylamino propionitrile in presence of nickel
ActiveCN102050742BHigh selectivityLow operating costOrganic compound preparationAmino compound preparationHydrogenAminopropionitrile
The invention relates to a method for preparing dimethylamino propylamine through hydrogenating dimethylamino propionitrile in the presence of nickel. The method comprises the following step: under the reaction condition thatdimethylamino propionitrile is hydrogenated to prepare dimethylamino propylamine in the presence of a catalyst, contacting dimethylamino propionitrile with hydrogen for reaction, wherein the catalyst contains at least one amorphous Ni-Al-Mo-M catalyst; by taking the weight of the catalyst as a reference, the weight ratio of Ni to Al to Mo to M is (5-500):(1-150):(1-150):1; and M is selected from one or more of transition metals in IB group, IIB group, IIIB group, IVB group, VIIB group, VIB group except for Mo and VIII group except for Ni. Compared with the prior art, the invention has the advantage that the selectivity of the dimethylamino propylamine prepared through hydrogenation by dimethylamino propionitrile is obviously increased.
Owner:CHINA PETROLEUM & CHEM CORP +1
One process of using two fixed bed reactors to continuously catalyze the N-methyl-1,3-propyleneramine
ActiveCN112961061BEasy to achieve serial productionLow costCarboxylic acid nitrile preparationOrganic compound preparationAminopropionitrileMeth-
The invention discloses a process for preparing N-methyl-1,3-propylenediamine through continuous catalytic reaction in two fixed-bed reactors, which belongs to the technical field of organic chemical industry. Using monomethylamine and acrylonitrile as raw materials, first continuously pass through the first fixed-bed reactor to react to generate 3-methylaminopropionitrile intermediate, and then continuously enter the second fixed-bed hydrogenation reactor for catalytic hydrogenation Hydrogen to generate N-methyl-1, 3-propylenediamine, yield ≥ 99%. The process of the invention is continuous production, simple, less three wastes, energy saving, environmental protection, low cost, and easy to realize industrialization.
Owner:DALIAN UNIV OF TECH
Weather-resistant advertisement sticker
ActiveCN114806445ANo change in adhesionExtended service lifeStampsNon-macromolecular adhesive additivesPolymer scienceMeth-
The weather-resistant advertisement sticker comprises a base material layer, a back adhesive layer and a release layer, one face of the base material layer is coated with the back adhesive layer, and the release layer is arranged on the face, opposite to the base material layer, of the back adhesive layer; the back adhesive layer is obtained by coating and drying an adhesive solution, and the adhesive solution is prepared from the following components in parts by weight: methyl methacrylate, styrene, 2-ethylhexyl acrylate, acrylic acid, bis (2, 4-dichlorobenzoyl) peroxide, azoisobutyronitrile, trimethylpentanediol, dimethylaminopropionitrile, a curing agent, deionized water, an emulsifier and hydroquinone. According to the weather-resistant advertisement sticker, the waterproof performance is improved, the adhesive force of the weather-resistant advertisement sticker is basically unchanged under the condition that the environment humidity is relatively high, and the weather-resistant advertisement sticker is stable in adhesion and does not fall off.
Owner:ZHEJIANG FULAI NEW MATERIAL CO LTD
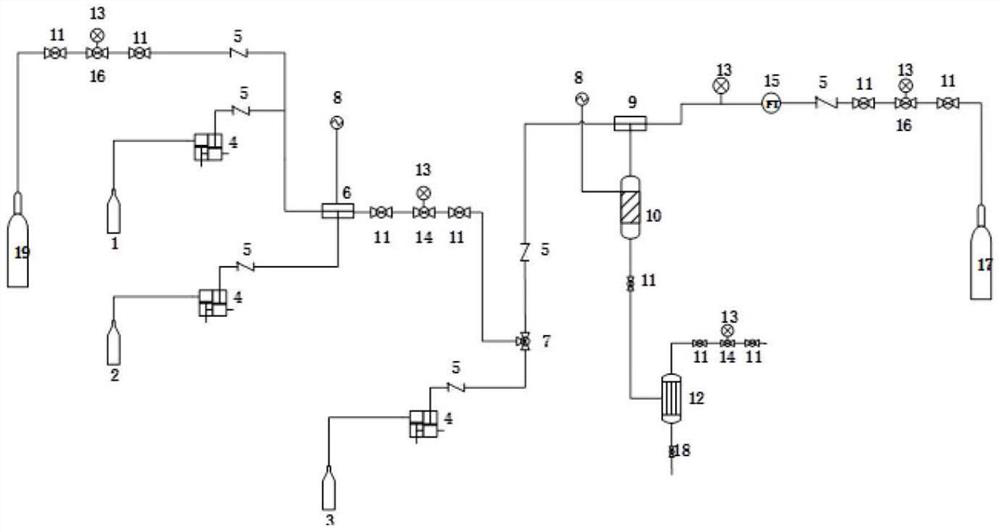
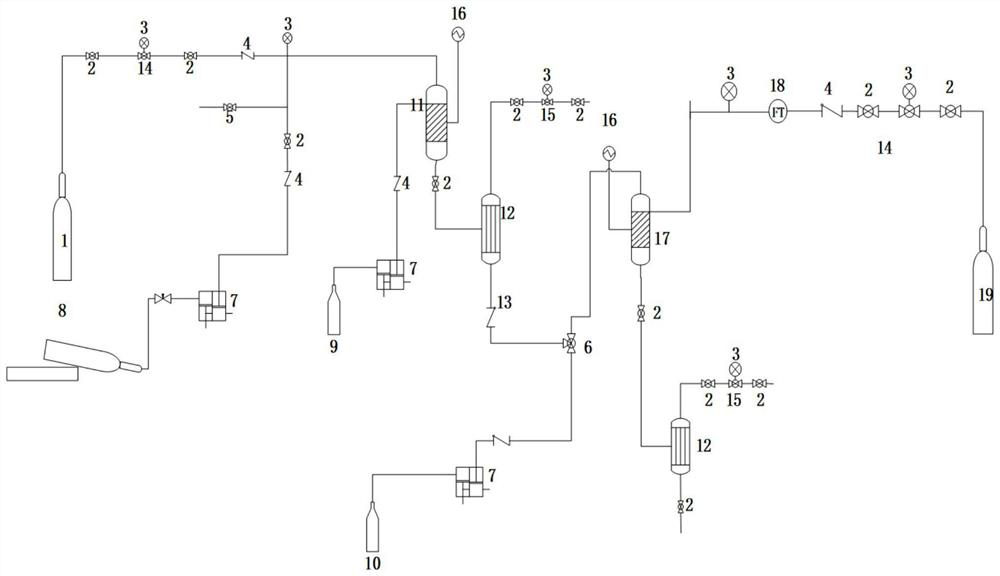
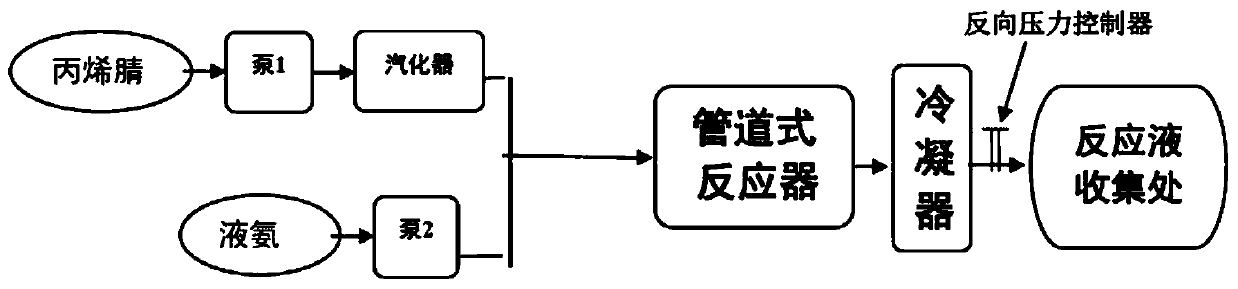
A kind of synthetic method and device of β-aminopropionitrile
ActiveCN109438283BHigh yieldReduce manufacturing costOrganic compound preparationDistillation separationChemical synthesisAminopropionitrile
The invention relates to the technical field of chemical synthesis technology, in particular to a synthesis method and device for β-aminopropionitrile. The synthetic method of described β-aminopropionitrile comprises the following steps: (a) after the reaction of acrylonitrile and ammonia water, distillation collects β-aminopropionitrile; (b) mixing the remaining waste liquid after distillation with ammonia water and raising the temperature to obtain a mixed Liquid, distill mixed solution to collect β-aminopropionitrile, reclaim distillation waste liquid and ammoniacal liquor reaction; Wherein, the distillation condition in the step (b) comprises: adopt steam distillation to distill mixed solution to collect β-aminopropionitrile, steam distillation comprises at least Two-stage steam distillation, the water vapor pressure gradient of each stage of steam distillation increases. The regulation of the process conditions in the present invention can effectively control the temperature of the distillation system not to exceed 120°C while ensuring the distillation yield of the product β-aminopropionitrile, and the material components in the waste liquid are not destroyed, and can be used as raw materials for the reaction. Improve the yield of β-aminopropionitrile.
Owner:江苏兄弟维生素有限公司
Preparation of Methyldopa by Hydrolysis of α-Methyl-(3,4-Dimethoxyphenyl)-α-aminopropionitrile by Two-step Hydrolysis
InactiveCN102786428BEmission reductionReduce energy costsOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisHigh concentration
Owner:ZHEJIANG UNIV
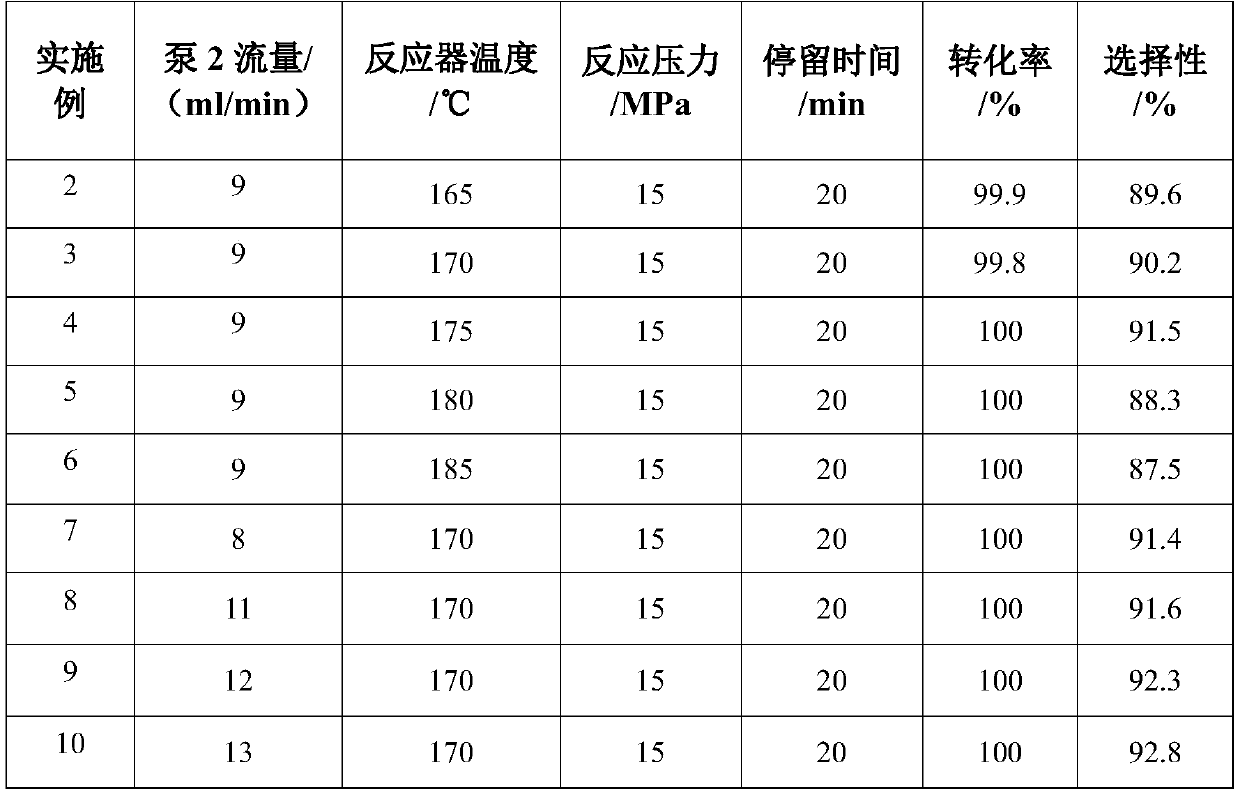
A kind of method preparing 3-aminopropionitrile under supercritical condition
ActiveCN107935888BReduce energy consumptionReduce generationCarboxylic acid nitrile preparationOrganic compound preparationAminopropionitrilePtru catalyst
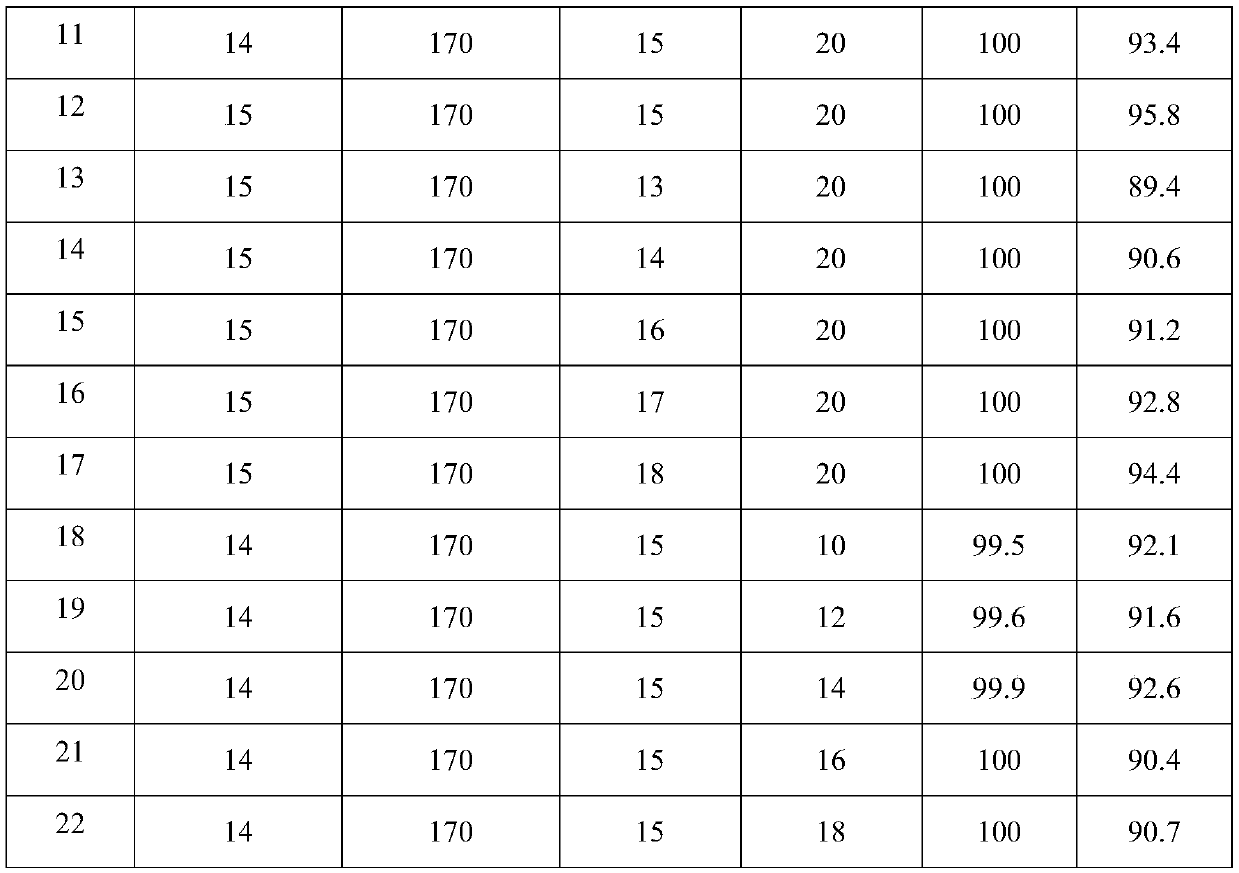
The invention discloses a method for preparing 3-aminopropionitrile under a supercritical condition. According to the method, liquid ammonia and acrylonitrile are used as raw materials which are subjected to direct addition reaction in a pipeline reactor without adding a catalyst and a solvent to obtain 3-aminopropionitrile. The reaction according to the invention is carried out under a supercritical condition of the liquid ammonia, thus avoiding the use of a catalysts and a solvent when the liquid ammonia is used in reports in the past; a product can be obtained through continuous rectification; unreacted raw materials can be directly used; the process is safe and environmentally friendly, substances of non-reactive raw materials are not introduced, and the method accords with a concept of green chemistry. The conversion rate of acrylonitrile can reach 99.5-100.0%, and the selectivity can reach 85.5-95.8%, so that the acrylonitrile has a high industrial application value.
Owner:ZHEJIANG NHU CO LTD +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com