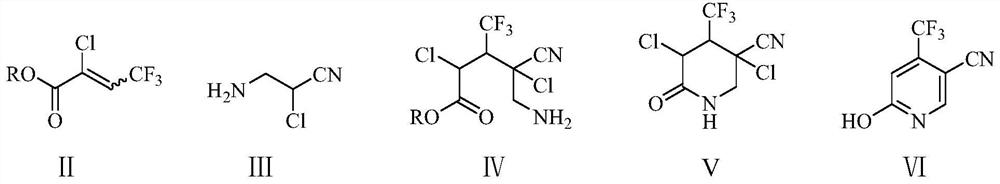
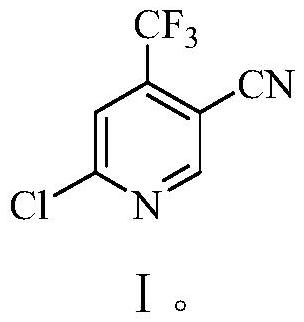
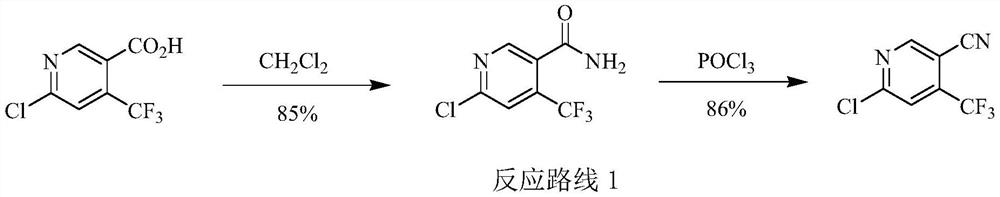
Preparation method of 6-chloro-4-trifluoromethyl-3-cyanopyridine
A technology of trifluoromethyl and cyanopyridine, applied in the field of preparation of 6-chloro-4-trifluoromethyl-3-cyanopyridine, can solve the problems of complicated operation, poor environmental protection, low yield and the like, and achieves The effect of high yield and purity, less by-products, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Preparation of 6-hydroxyl-4-trifluoromethyl-3-cyanopyridine (Ⅵ)
[0054] In the 250 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, add 18.9 grams (0.1 moles) 2-chloro-3-trifluoromethyl methacrylate, 10.5 grams (0.1 moles) 2-chloro-3 -Aminopropionitrile, 0.04 g of DBU, stirred and reacted between 20 and 25°C for 3 hours, the addition reaction was detected by GC and transferred to a constant pressure dropping funnel for use. Add 100 g of toluene to a 500 ml four-neck flask connected with a stirring, thermometer, reflux condenser and constant pressure dropping funnel, heat, and add the obtained addition product dropwise at 90 to 95 ° C, dropwise for 2 hours After that, react at 90 to 95°C for 2 hours, cool, add 34.5 grams of potassium carbonate between 20 and 25°C, stir and react for 3 hours between 20 and 25°C, pour the reactant into 100 grams of water, and acidify with 30% hydrochloric acid The pH value of the sys...
Embodiment 2
[0058] Example 2: Preparation of 6-hydroxyl-4-trifluoromethyl-3-cyanopyridine (Ⅵ)
[0059] In a 500 ml four-necked flask connected with stirring, a thermometer, and a reflux condenser, add 101.3 grams (0.5 moles) of 2-chloro-3-trifluoroethyl methacrylate, 53.0 grams (0.51 moles) of 2-chloro-3 -Aminopropionitrile, 0.5 g of DBU, stirred and reacted at 20-25°C for 3 hours, the addition reaction was detected by GC and transferred to a constant pressure dropping funnel for use. Add 400 g of toluene to a 1000 ml four-neck flask connected with a stirring, thermometer, reflux condenser and constant pressure dropping funnel, heat, and add the obtained addition product dropwise at 90 to 95 ° C, dropwise for 2 hours After that, react at 90 to 95°C for 2 hours, cool, add 45 grams of sodium hydroxide between 20 and 25°C, stir and react for 3 hours between 20 and 25°C, pour the reactant into 200 grams of water, and wash with 30% hydrochloric acid The pH value of the acidification system wa...
Embodiment 3
[0060] Embodiment 3: Preparation of 6-chloro-4-trifluoromethyl-3-cyanopyridine (I)
[0061] Add 150 grams of 1,2-dichloroethane, 18.8 grams (0.1 moles) of 6-hydroxyl-4-trifluoromethane prepared in Example 1 to a 500-milliliter four-necked flask equipped with a thermometer, mechanical stirring, and a reflux condenser. Base-3-cyanopyridine, 26.0 grams (0.125 moles) phosphorus pentachloride, 90-95 ℃ stirred and reacted for 10 hours, then slowly reactant was poured into 100 grams of ice water, fully stirred, then with 40wt% hydroxide Sodium aqueous solution neutralizes the pH value to 7-8, separates the layers, extracts the aqueous layer three times with 1,2-dichloroethane, 50 grams each time, combines the organic phases, washes with 30 grams of saturated saline, and then washes them with 5 grams of anhydrous Dry over sodium sulfate, and remove the solvent by rotary evaporation to obtain 19.4 g of 6-chloro-4-trifluoromethyl-3-cyanopyridine (I), with a yield of 93.9% and a gas phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com