Process for continuously preparing N-hydroxyethyl-1, 3-propane diamine by using micro-mixing and fixed bed reactor
A fixed bed reactor and hydroxyethyl technology, which is applied in the preparation of organic compounds, amino hydroxyl compounds, carboxylic acid nitriles, etc., can solve the problems of low production efficiency, many by-products, complex processes, etc., and improve efficiency , low cost, improve the effect of mass transfer and heat transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
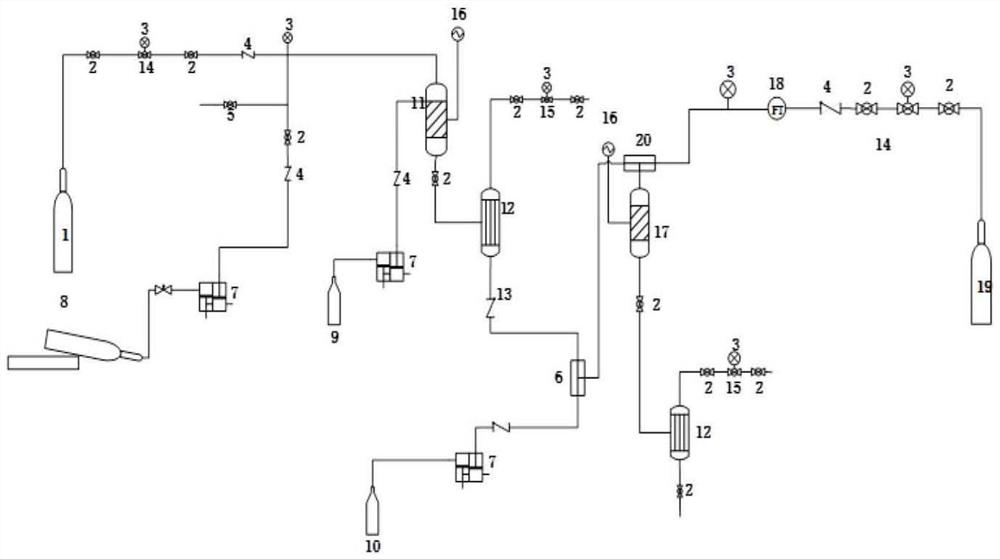
[0023] (1) Firstly, the reactor 11 and the reactor 17 are filled with catalyst 4A type molecular sieve and Raney-Ni respectively. (2) Open the nitrogen cylinder 1, purge the whole system, discharge the air, set the pressure of the fixed bed reactor 11 through the nitrogen pressure reducing valve 14: 3MPa, and set the temperature by the temperature controller 16: 35°C. The pressure of the fixed bed reactor 17 is set by the hydrogen cylinder 19 through the pressure reducing valve 14 to the system: 3MPa, and the hydrogen flow rate is controlled by the mass flow controller 18, and the temperature is set by the temperature controller 16 to 65°C. (3) Use two double-plunger micropumps 7 to transport ethanolamine (99%) and acrylonitrile (99%) to the fixed-bed reactor 11 filled with 4A type molecular sieve catalyst in a molar ratio of 1.1:1 for reaction, At an airspeed of 0.75h -1 Under the condition, continuous reaction makes the reaction solution that contains N-hydroxyethyl-3-amino...
Embodiment 2
[0025] Using the production method of Example 1, the difference is that the ethanolamine ethanol solution with a concentration of 80% is used, the concentration of acrylonitrile is 99%, the molar ratio of ethanolamine to acrylonitrile is 2:1, the reaction temperature is 50° C., and the reaction pressure is 4 MPa. speed is 1h -1 , the catalyst is 3A molecular sieve, the catalyst for hydrogenation is: 5% NaOH ethanol solution, the content of N-hydroxyethyl-3-aminopropionitrile in the hydrogenation raw material is 30%, and the content of NaOH is 0.5%; hydrogen and N-hydroxyethyl - The molar ratio of 3-aminopropionitrile is 10:1, the reaction temperature is 70°C, the reaction pressure is 4MPa, and the space velocity is 0.8h -1 , the hydrogenation catalyst is Raney Ni, the conversion rate of acrylonitrile is 99.8%, and the yield rate of N-hydroxyethyl-1,3-propanediamine is 99.2%.
Embodiment 3
[0027] Adopt the production method of Example 1, the difference is that the ethanolamine methanol solution with a concentration of 60% is used, the concentration of acrylonitrile is 99%, the molar ratio of ethanolamine to acrylonitrile is 1.2:1, the reaction temperature is 25°C, and the reaction pressure is 2MPa. Residence time 1.2h -1 , the catalyst is 5A molecular sieve, the catalyst for hydrogenation is: methanol solution of 10% NaOH, the content of N-hydroxyethyl-3-aminopropionitrile in the hydrogenation raw material is 50%, the content of NaOH is 2%, hydrogen and N-hydroxyethyl -The molar ratio of 3-aminopropionitrile is 15:1, the reaction temperature is 55°C, the reaction pressure is 3MPa, and the space velocity is 1.1h -1 , the hydrogenation catalyst is Raney Ni, the conversion rate of acrylonitrile is 99.8%, and the yield of N-hydroxyethyl-1,3-propanediamine is 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com