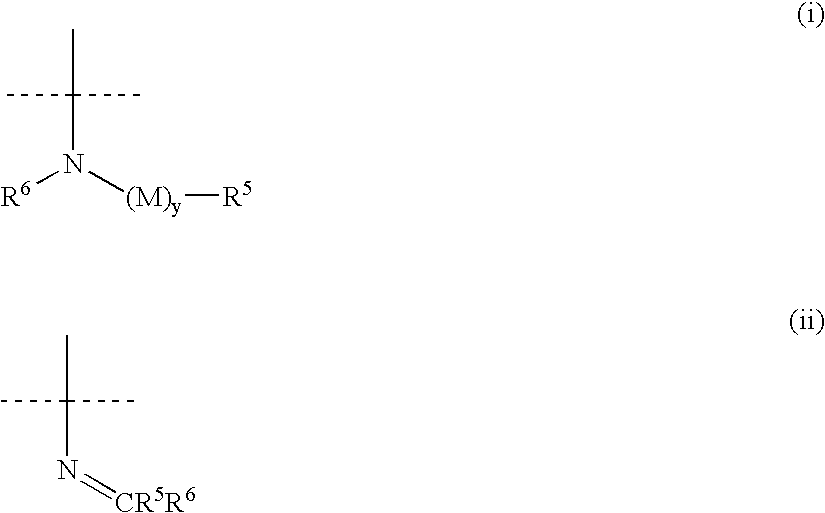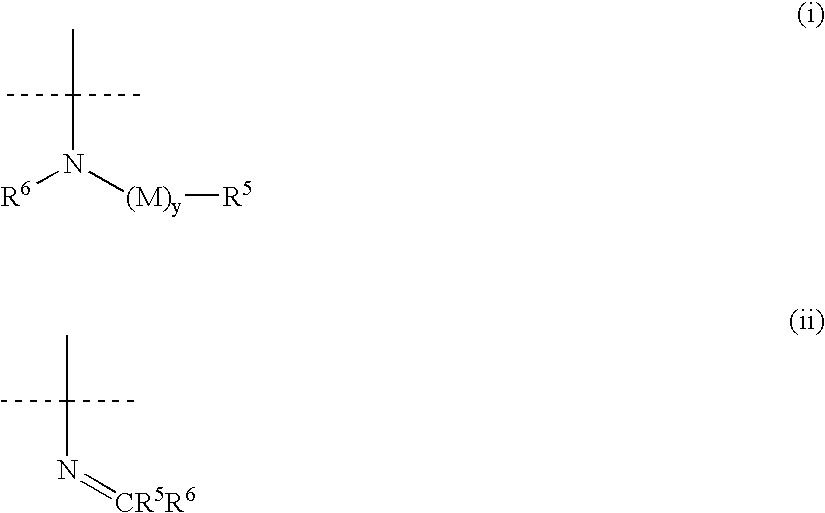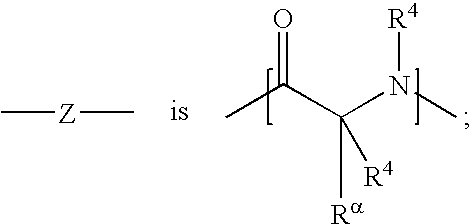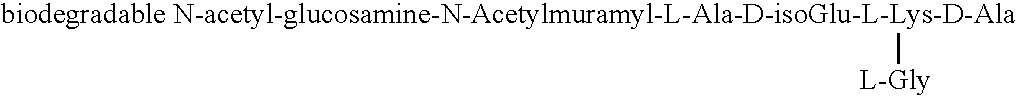Patents
Literature
51 results about "Cytoprotective Agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Agents administered before, with, or after cancer therapy to reduce or prevent damage or toxicity to the normal cells. (NCI)
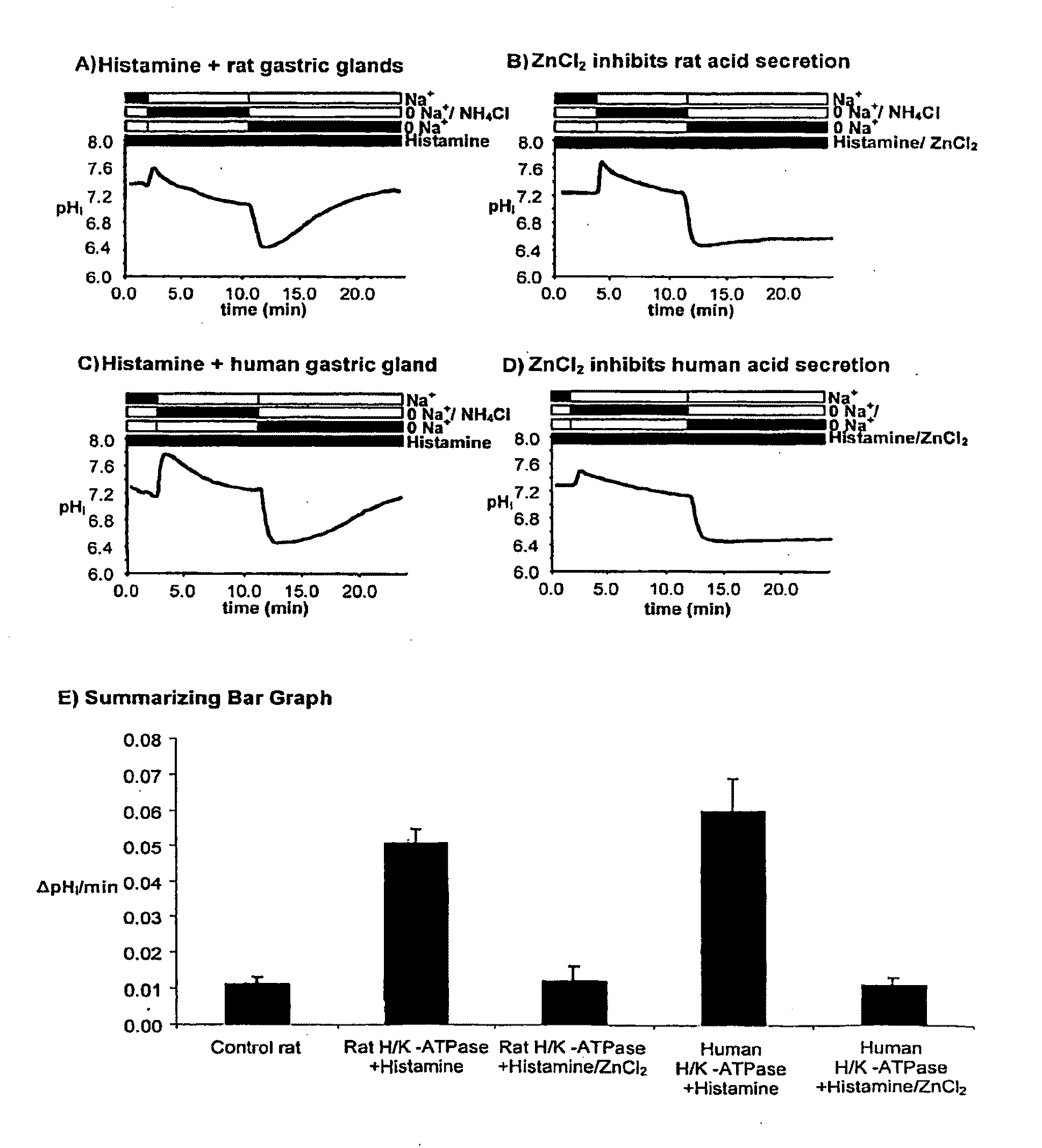
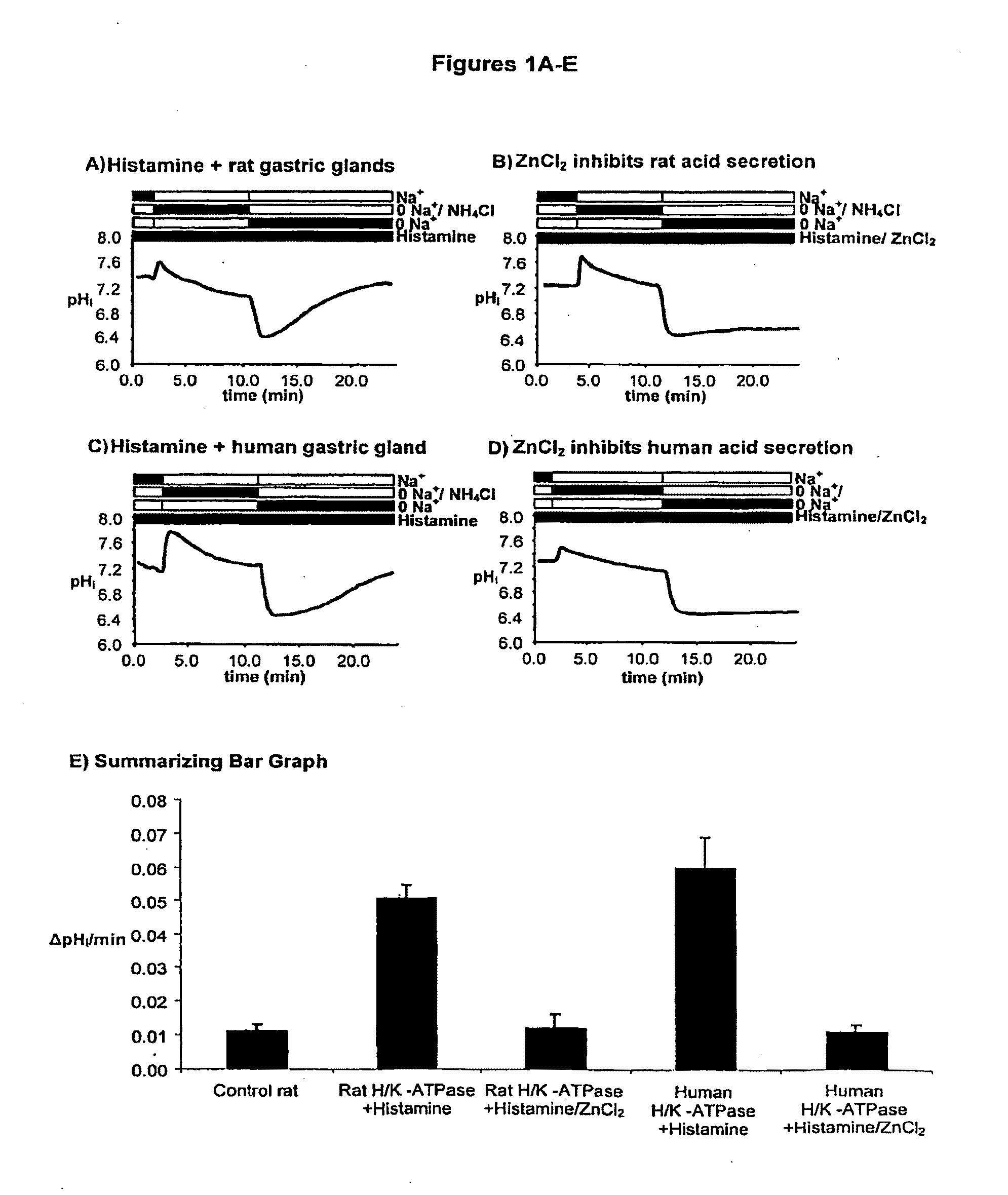
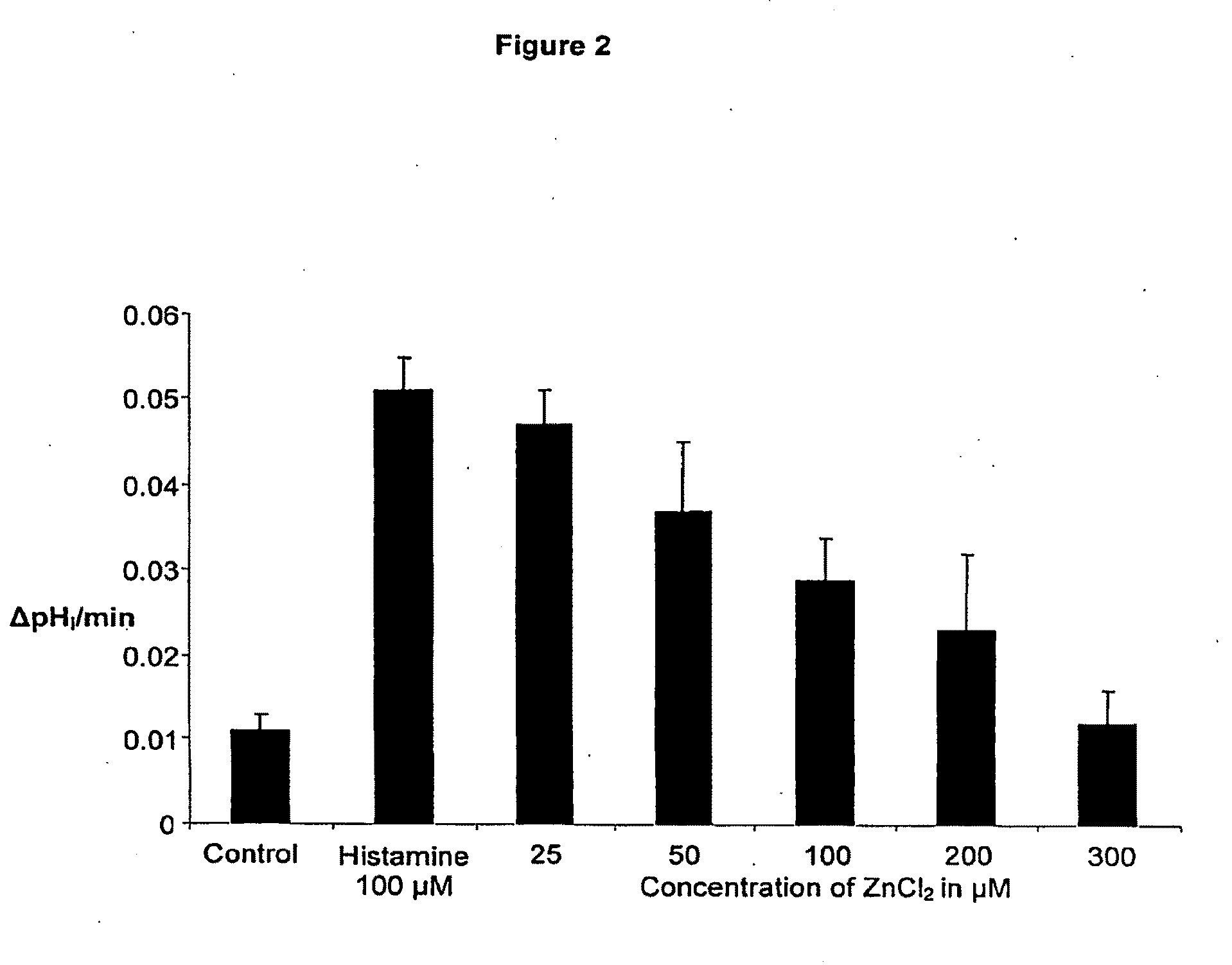
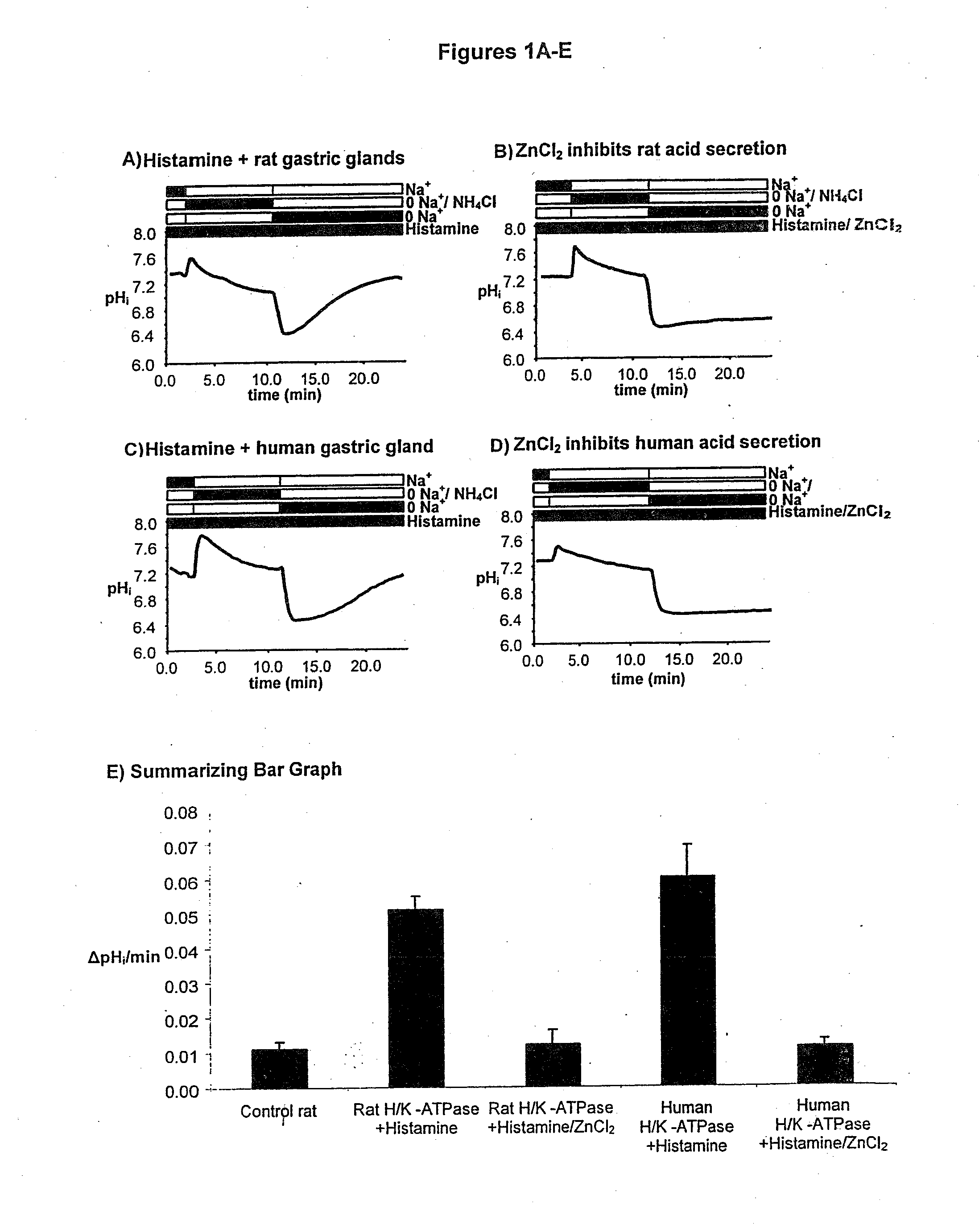
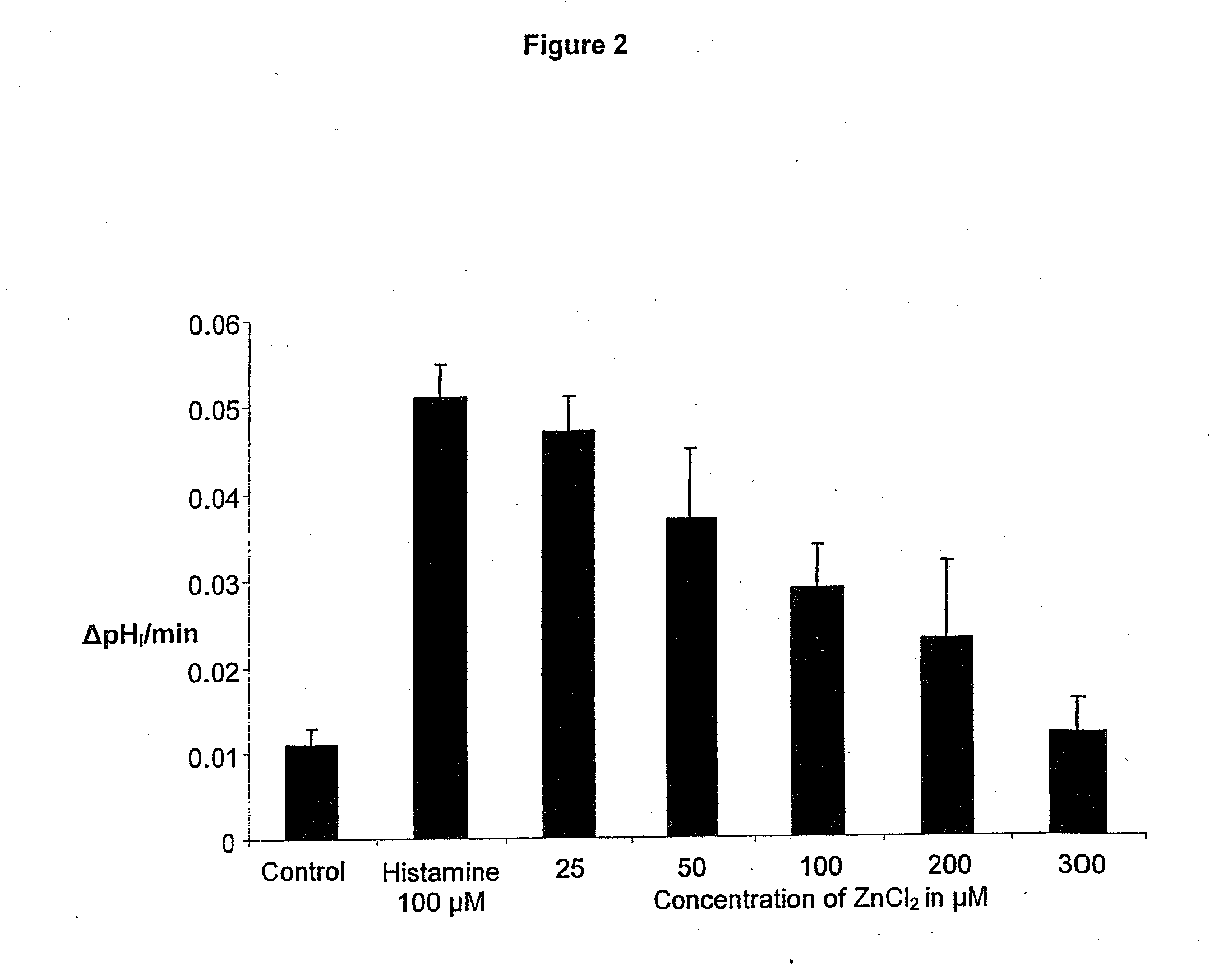
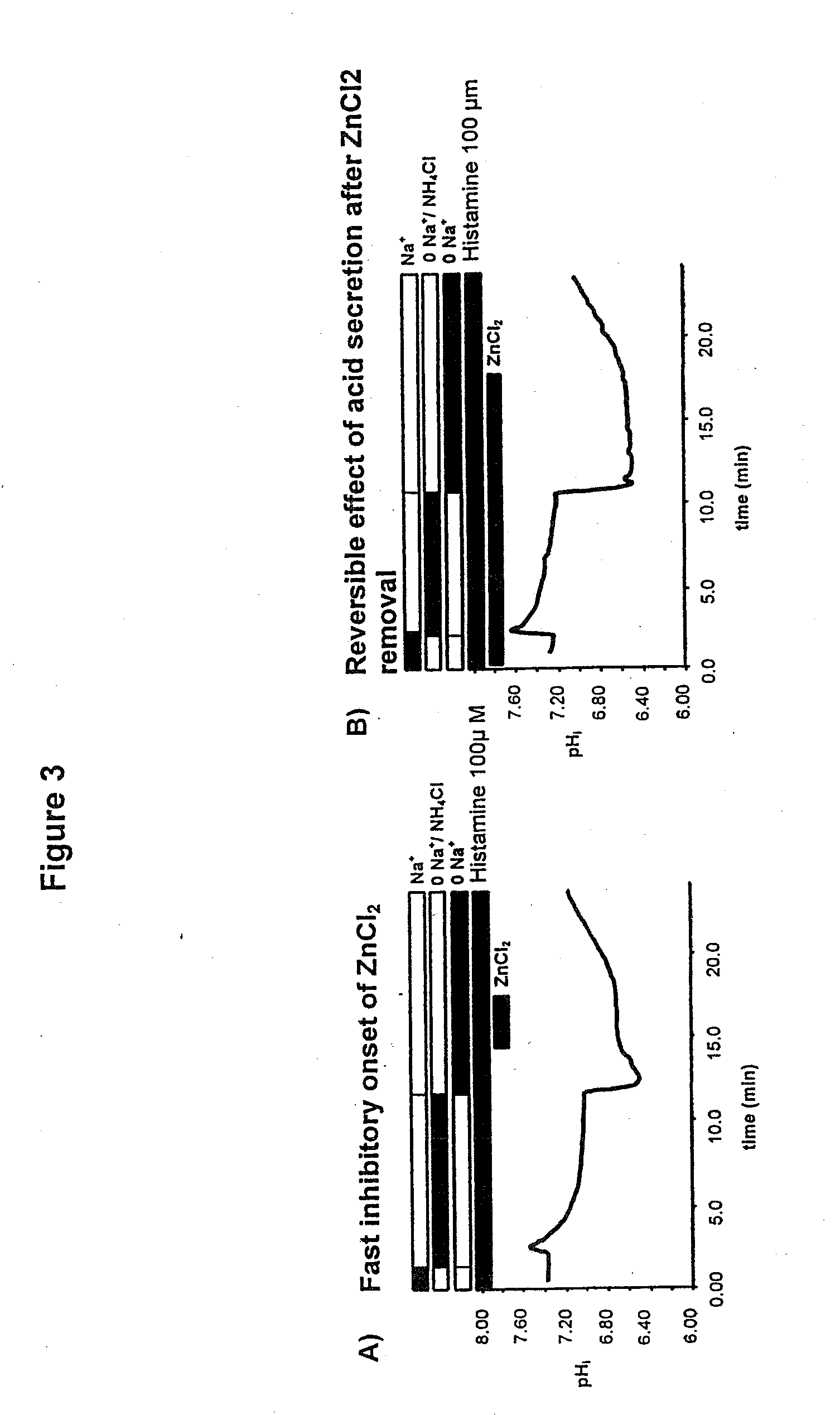
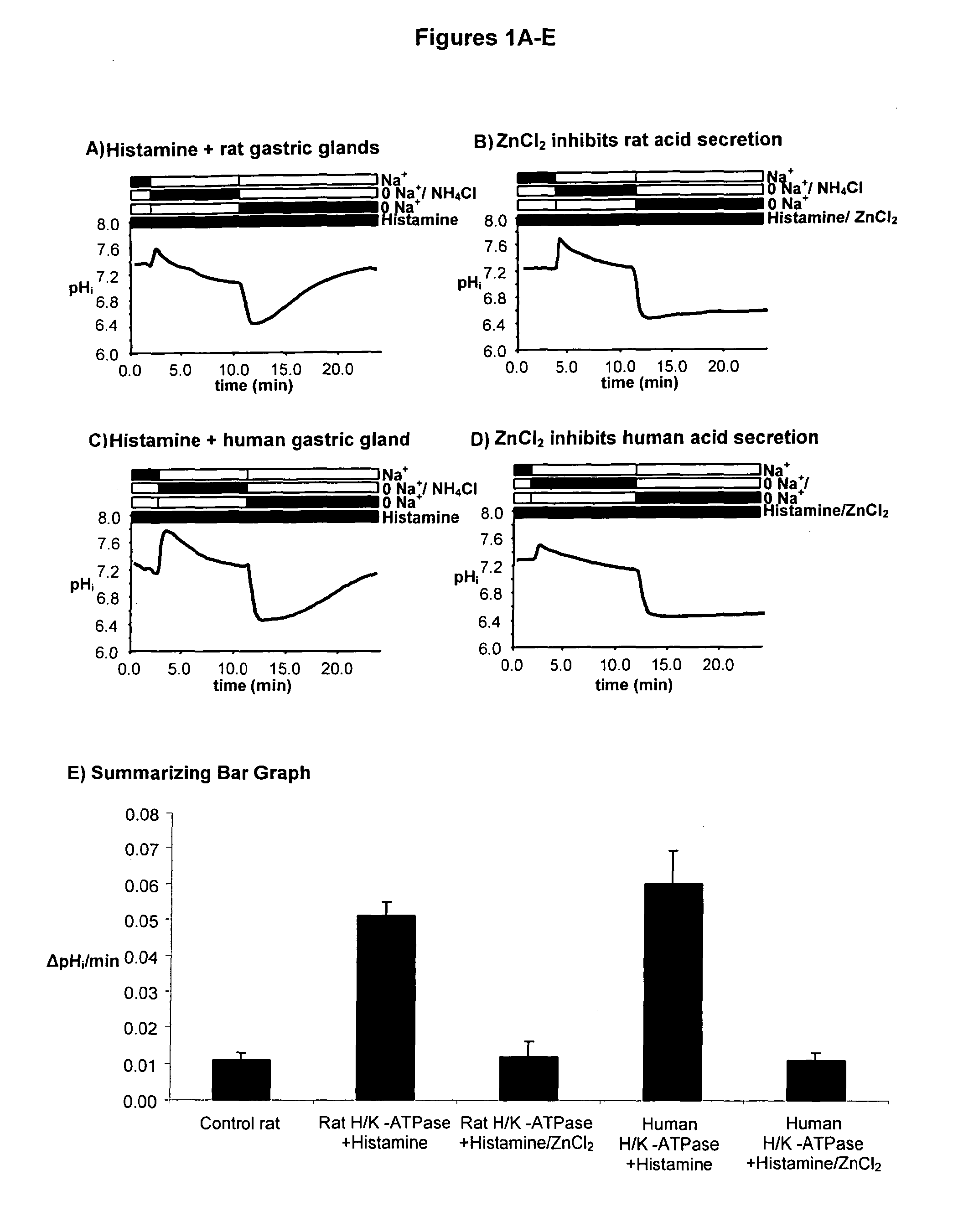
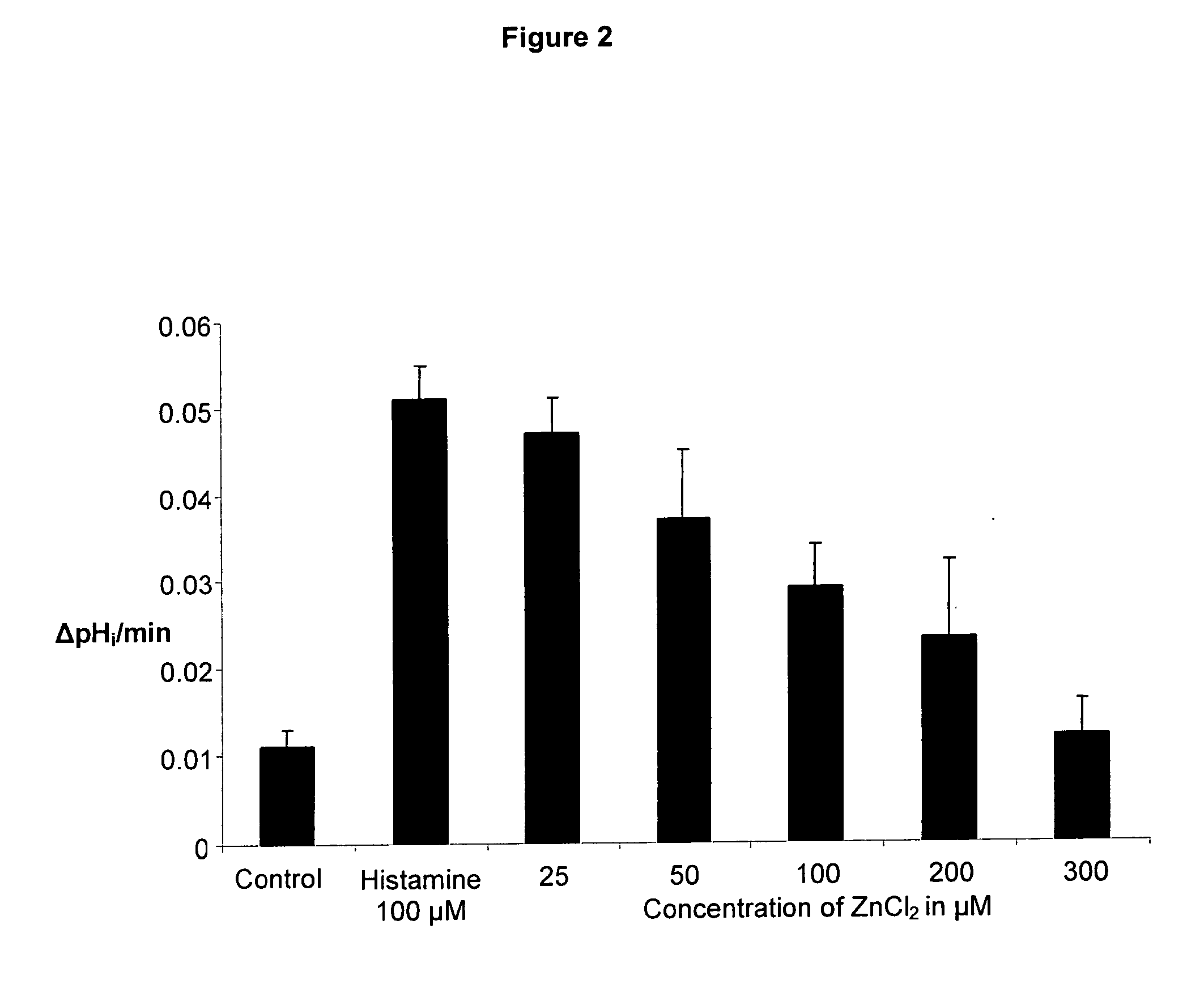
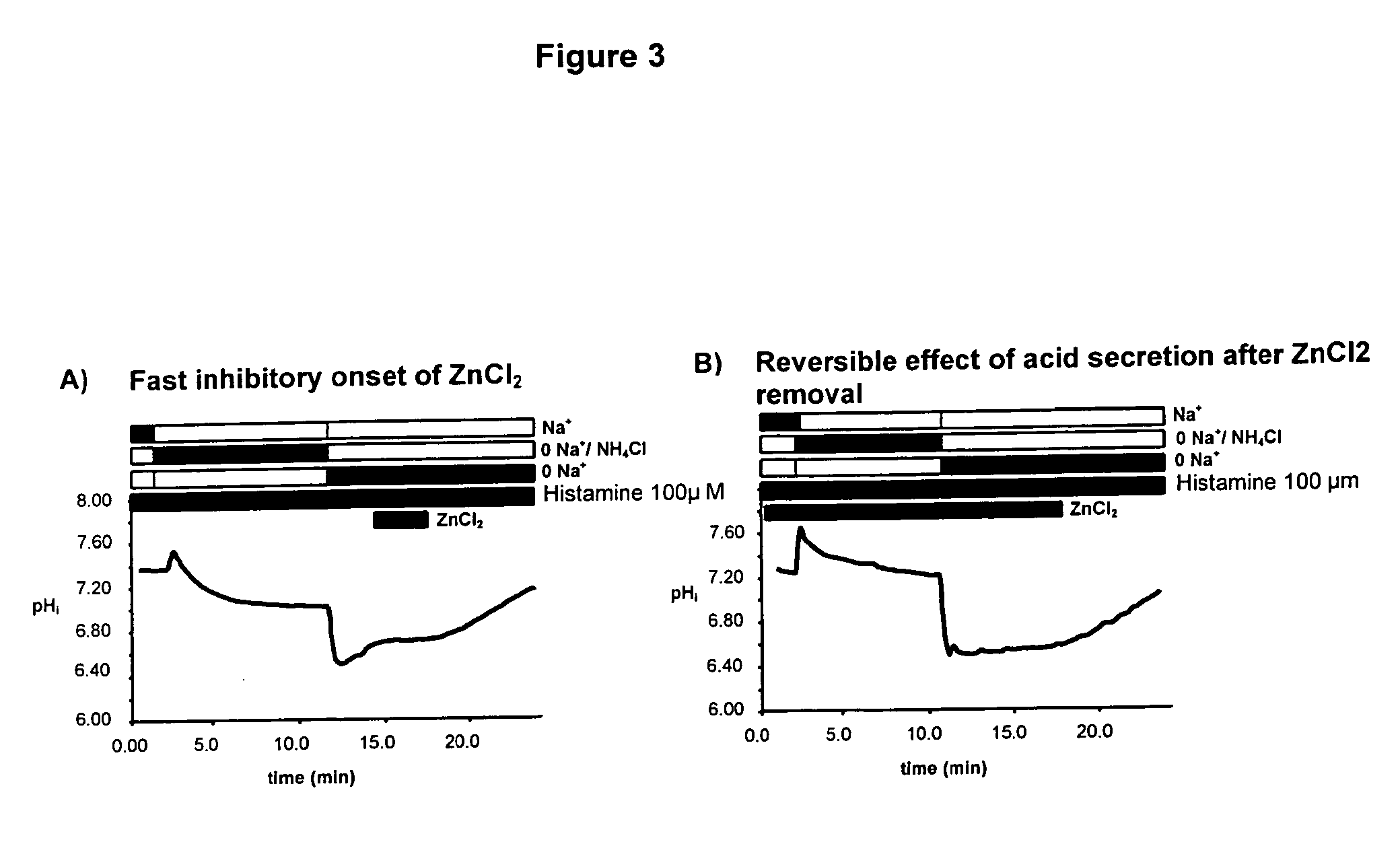
Compostions with enhanced bioavailability and fast acting inhibitor or gastric acid secretion
The present invention relates to the use of pharmaceutically acceptable zinc salts, preferably water soluble zinc salts alone or optionally, in combination with one or more of a protein pump inhibitor (PPI), H2 blocker, anti-H. pylori antibiotic / antimicrobial, cytoprotective agent or a combination agent as otherwise described herein for providing fast action with optional long duration effect in reducing gastric acid secretion, raising the pH of the stomach during resting phase as well as decreasing the duration of stomach acid release during a secretagogue phase and for treating conditions including gastroesophageal reflux disease (GERD), non-erosive reflux disease (NERD), Zollinger-Ellison syndrome (ZE disease), ulcer disease, and gastric cancer, as well as preventing or reducing the likelihood of ulcer disease. In addition, the present methods are useful for treating patients who are non-responsive to proton pump inhibitors (PPI) and as an alternative to traditional therapies or conditions which are caused by rapid and complete inhibition of secretagogue induced acid secretion. The present invention also relates to the use of one or more water soluble zinc salts, administered in combination with a therapeutic compound or agent (second therapeutic agent) which may be delivered orally with enhanced bioavailability (compared to compounds which are administered in the absence of water soluble zinc salts) or other favorable benefits. In addition, therapeutic agents which exhibit sensitivity to low pH may be advantageously orally administered in combination with an effective amount of at least one water soluble zinc salt. Compositions according to the present invention exhibit greater bioavailability of the active agent when formulated in combination with a water soluble zinc salt in oral dosage form than when administered with the water soluble zinc salt.
Owner:YALE UNIV
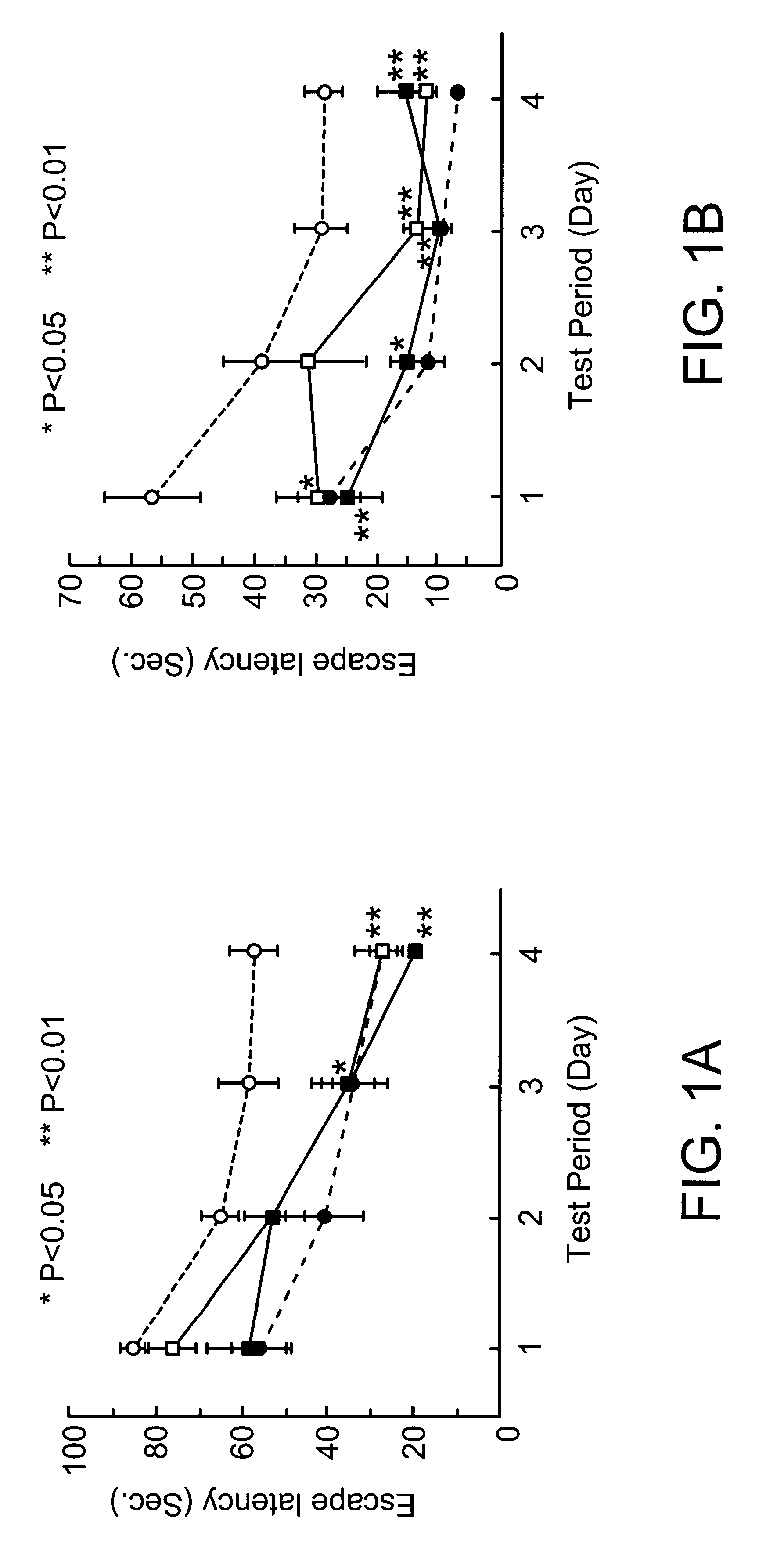
[1,2,3] triazolyl substituted quinolines and coumarins as inhibitors of leukotriene biosynthesis
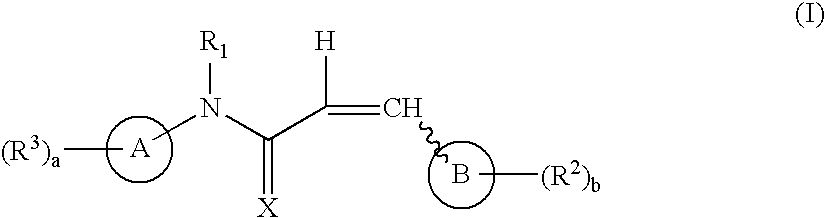
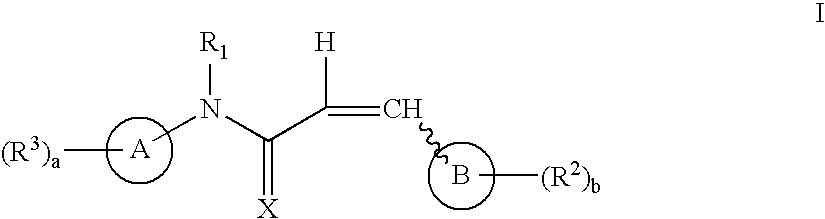
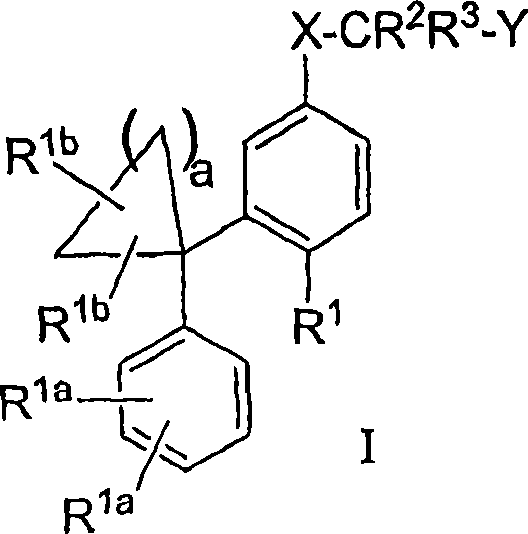
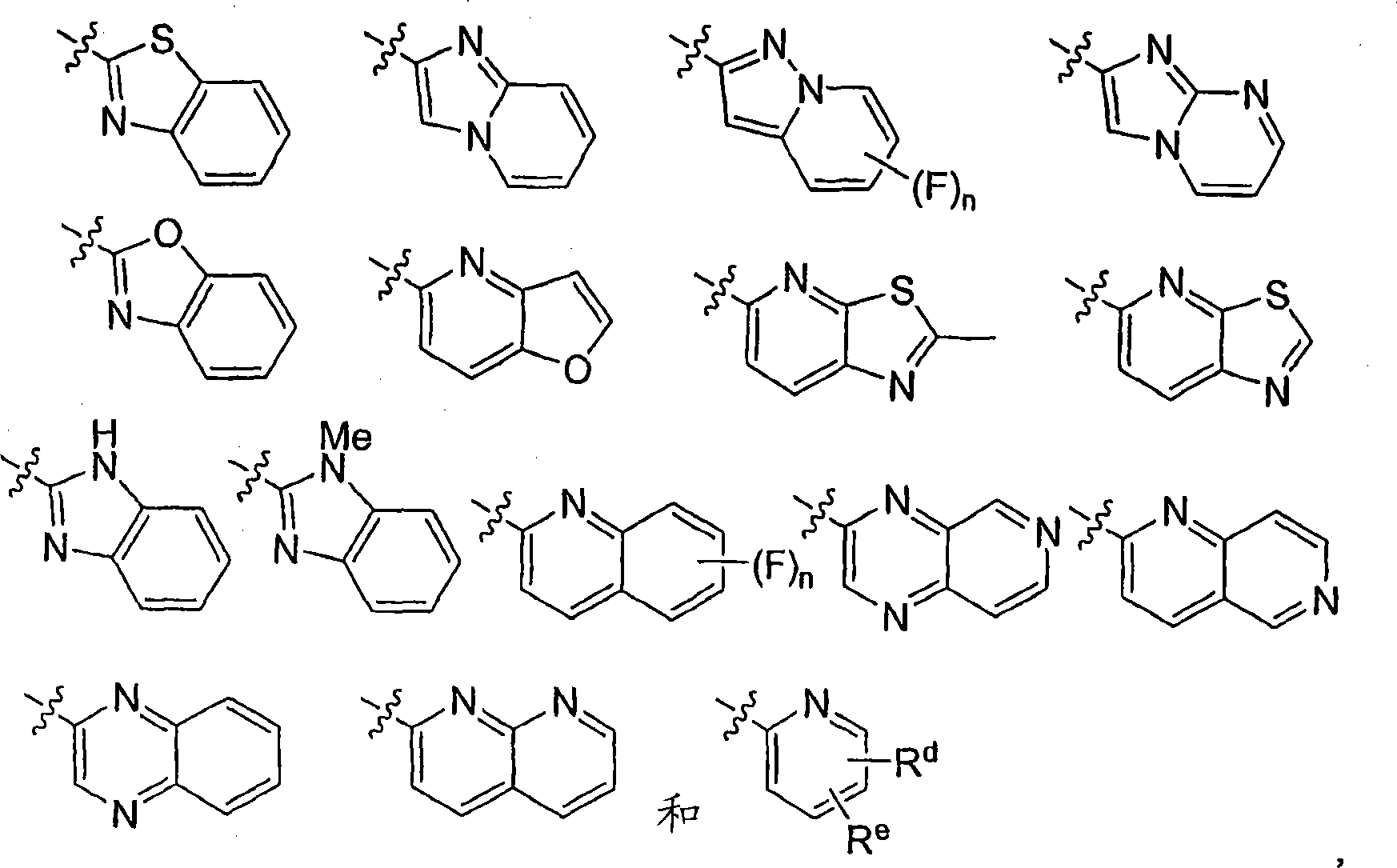
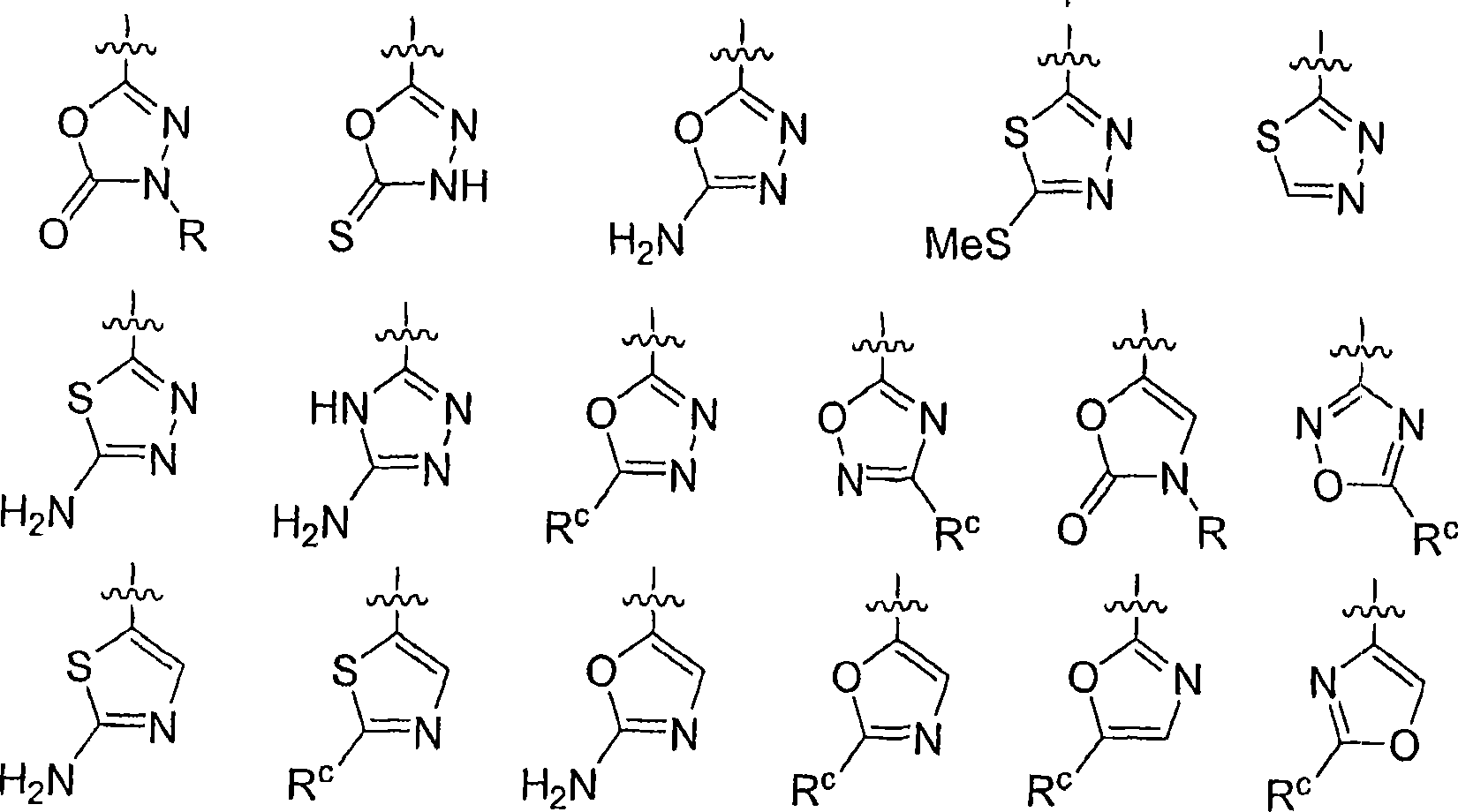
The instant invention provides compounds of Formula (I) which are leukotriene biosynthesis inhibitors. Compounds of Formula (I) are useful as anti-atherosclerotic, anti-asthmatic, anti-allergic, anti-inflammatory and cytoprotective agents.
Owner:MERCK CANADA
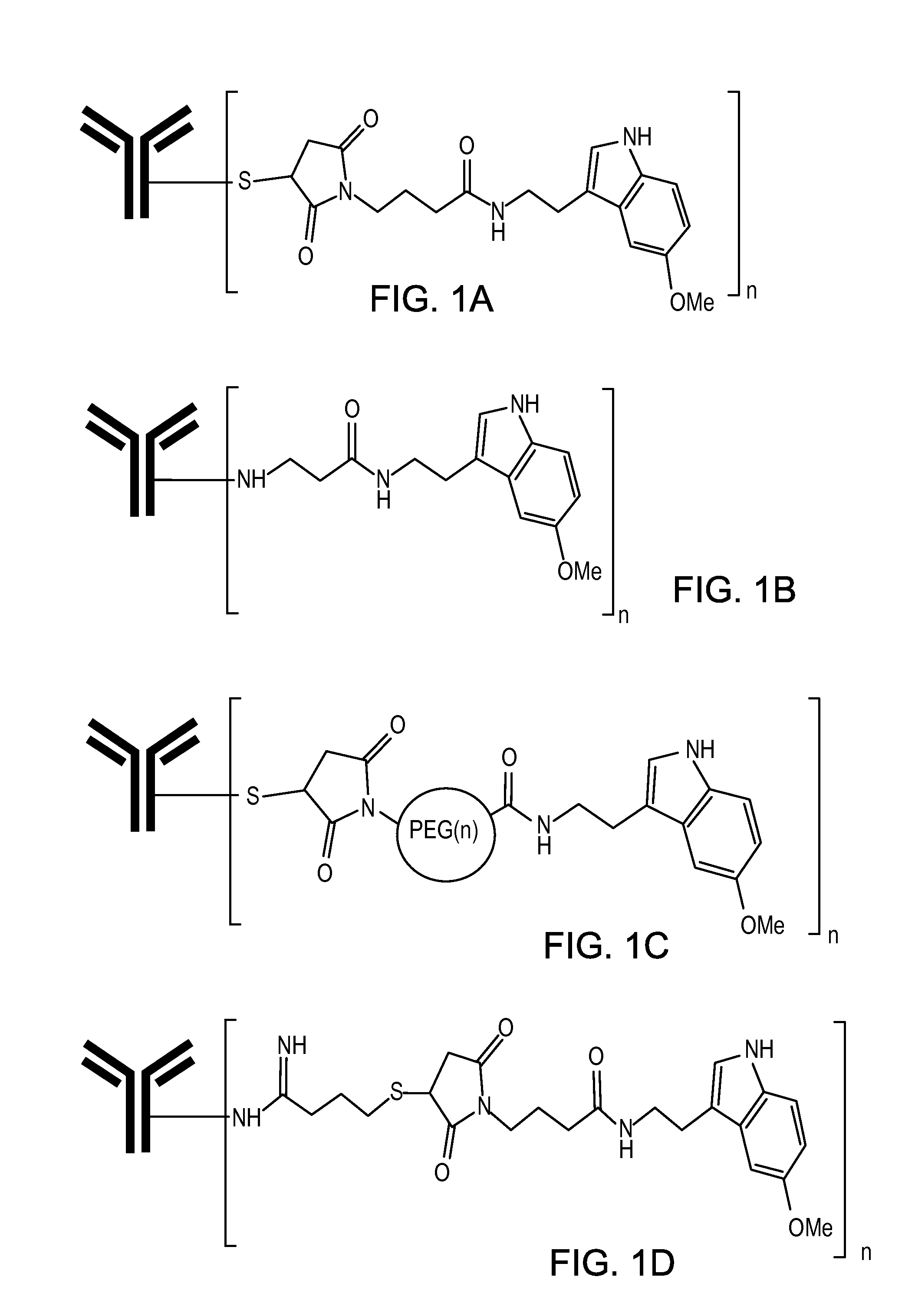
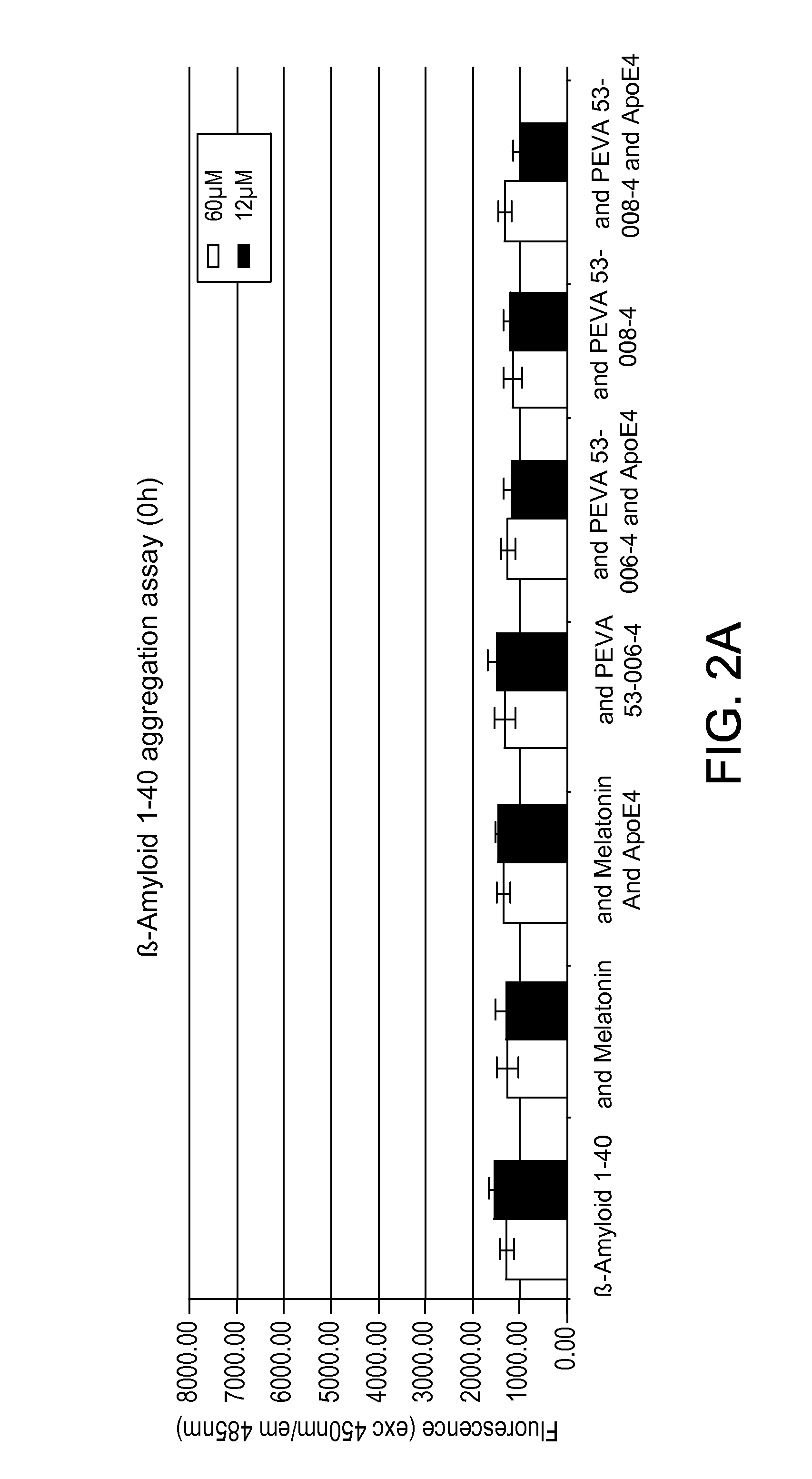
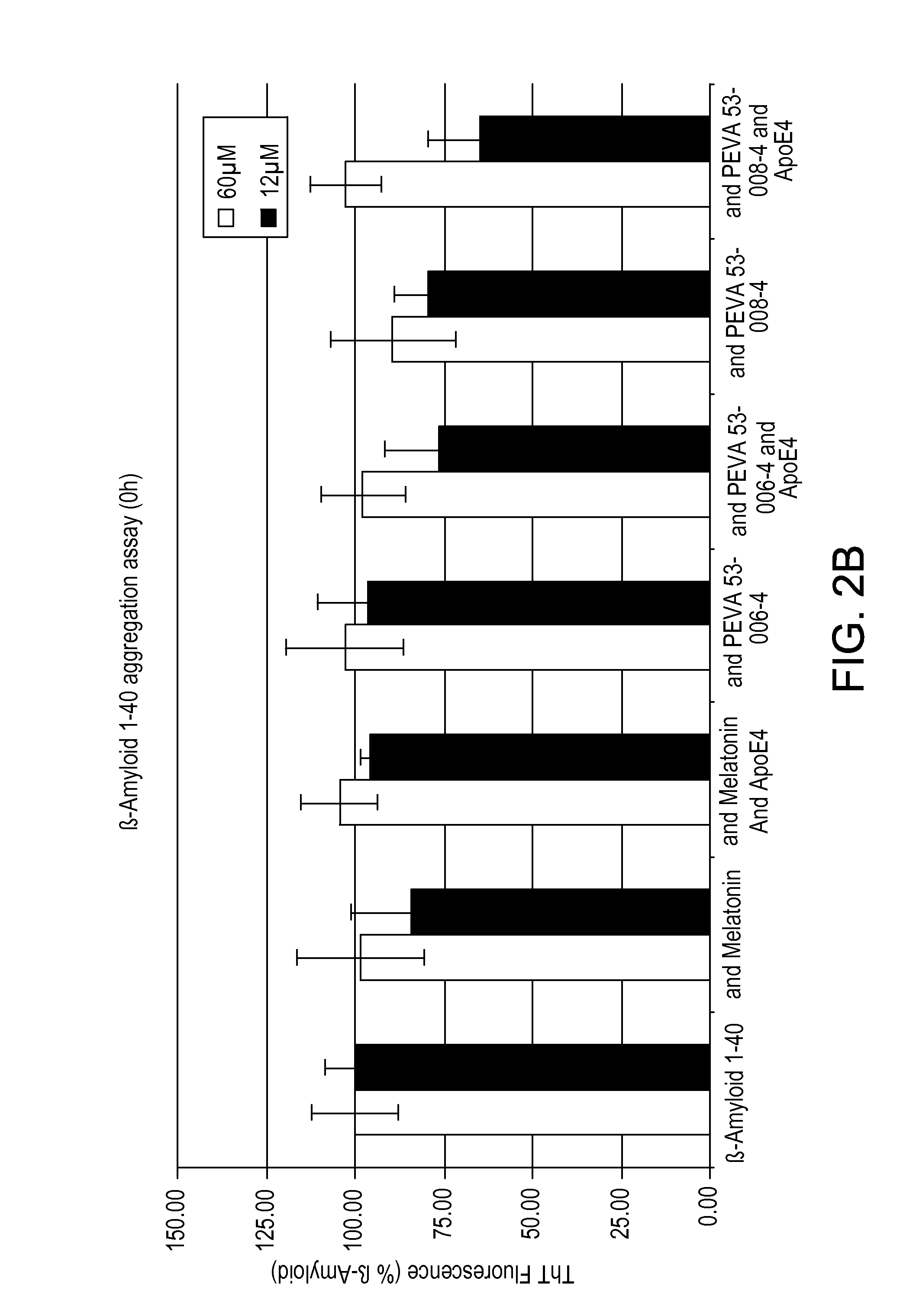
Compositions and methods for treatment of proteinopathies
InactiveUS20140294724A1Reduce inflammationReduce oxidative stressUltrasonic/sonic/infrasonic diagnosticsCompounds screening/testingDiseaseAntibody
Owner:INTELLECT NEUROSCI
Fast acting inhibitor of gastric acid secretion
ActiveUS20090035393A1Little to no potential for side effectRapid inhibitionAntibacterial agentsBiocidePresent methodAntibiotic Y
The present invention relates to the use of pharmaceutically acceptable zinc salts, preferably water soluble zinc salts alone or optionally, in combination with one or more of a protein pump inhibitor (PPI), H2 blocker, anti-H. pylori antibiotic / antimicrobial, cytoprotective agent or a combination agent as otherwise described herein for providing fast action with optional long duration effect in reducing gastric acid secretion, raising the pH of the stomach during resting phase as well as decreasing the duration of stomach acid release during a secretagogue phase and for treating conditions including gastroesophageal reflux disease (GERD), non-erosive reflux disease (NERD), Zollinger-Ellison syndrome (ZE disease), ulcer disease, and gastric cancer, as well as preventing or reducing the likelihood of ulcer disease. In addition, the present methods are useful for treating patients who are non-responsive to proton pump inhibitors (PPI) and as an alternative to traditional therapies or conditions which are caused by rapid and complete inhibition of secretagogue induced acid secretion.
Owner:GEIBEL JOHN P +1
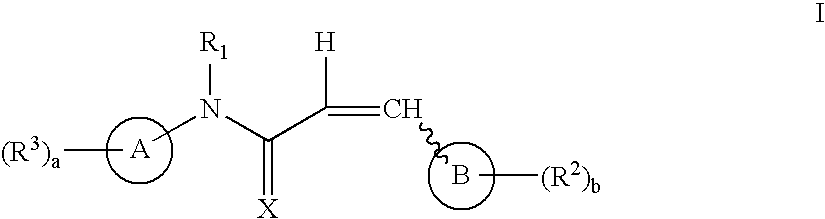
Aryl and heteroaryl propene amides, derivatives thereof and therapeutic uses thereof
InactiveUS20060167317A1Increase doseReduce and eliminate effectOrganic active ingredientsOrganic chemistryArylAnti-Carcinogenic Agents
Compounds useful as antiproliferative agents, radioprotective agents and cytoprotective agents, including, for example, anticancer agents, are provided according to formula I: wherein ring A, ring B,,X, R1, R2, R3, a, and b are as defined herein.
Owner:TEMPLE UNIVERSITY
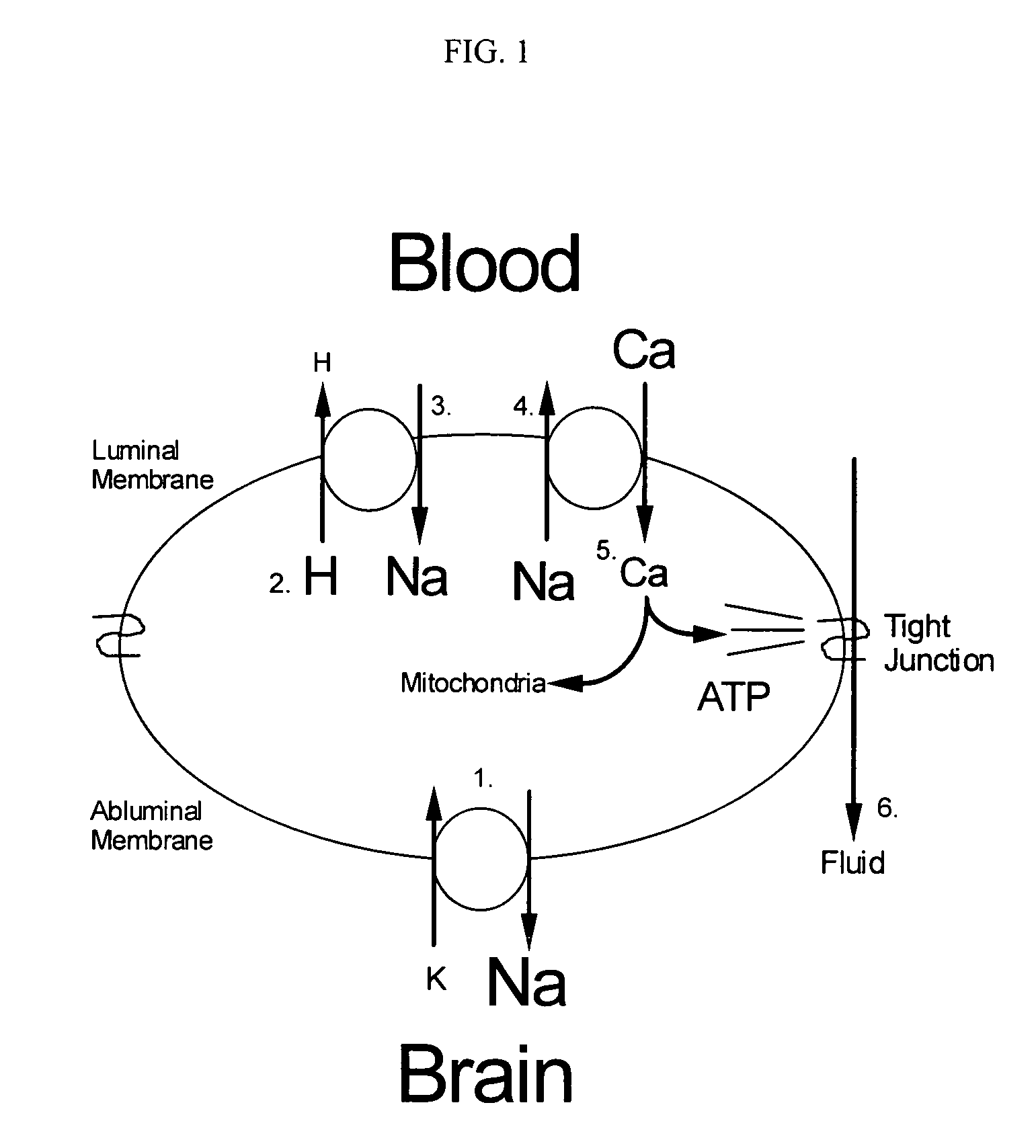
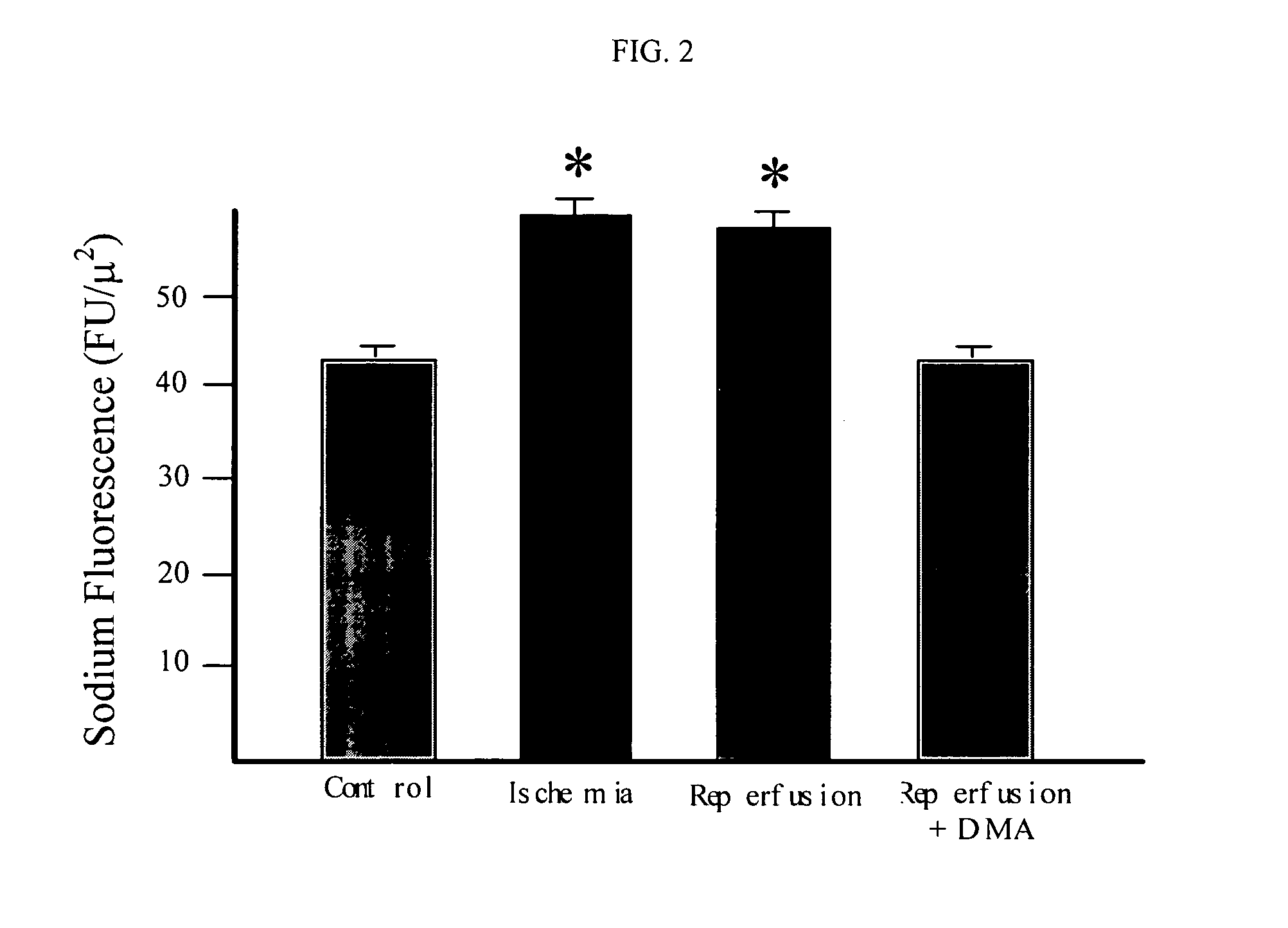
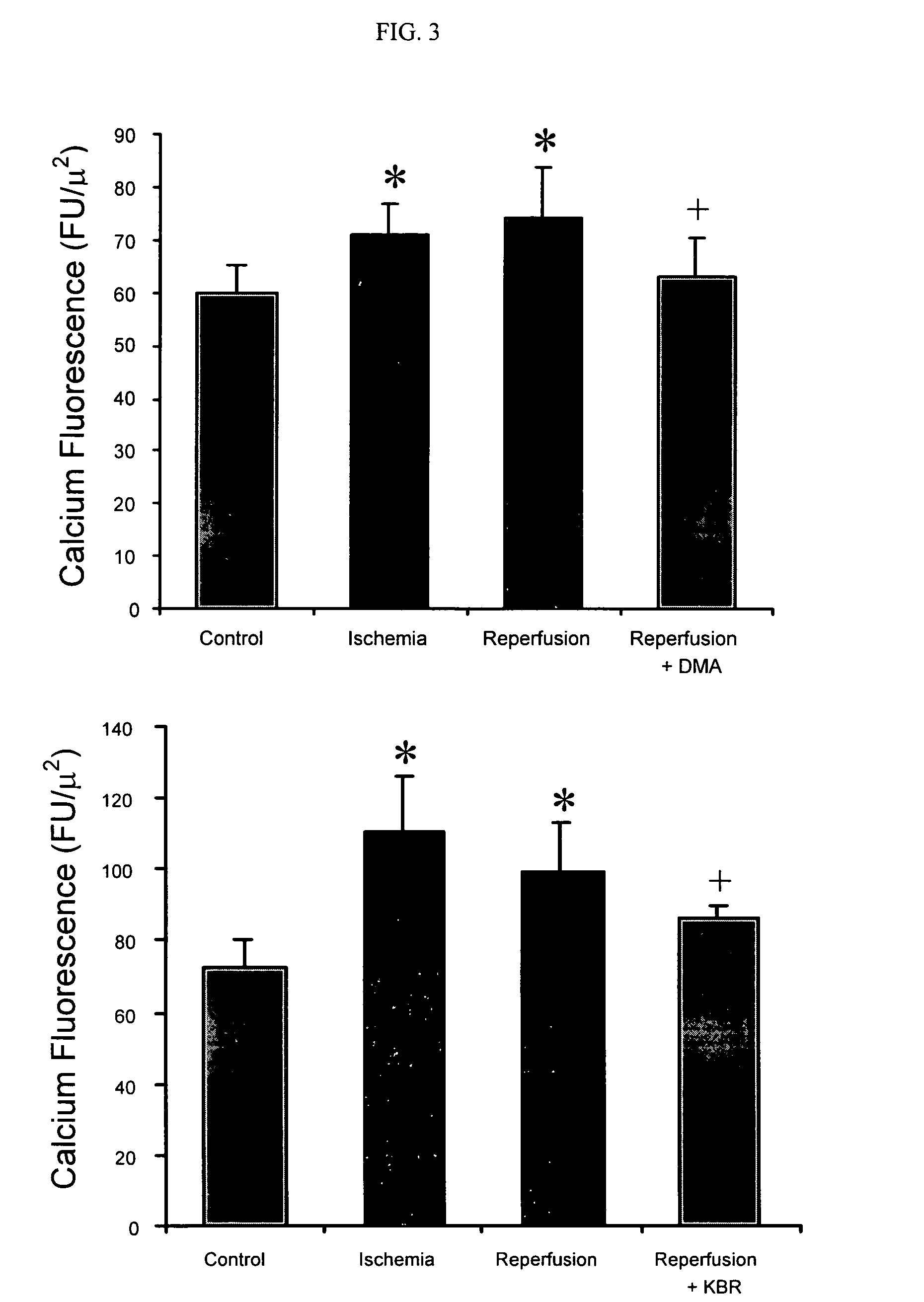
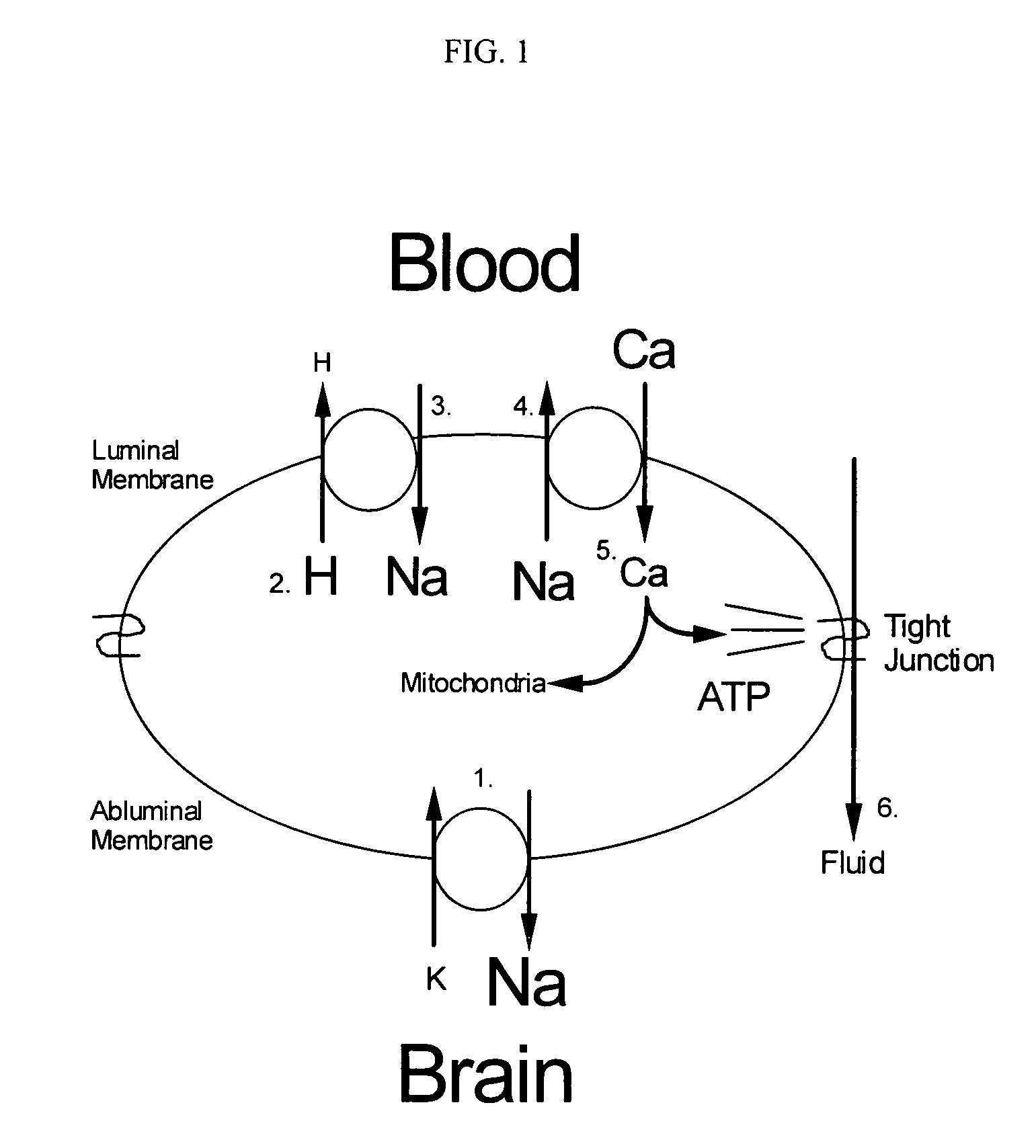
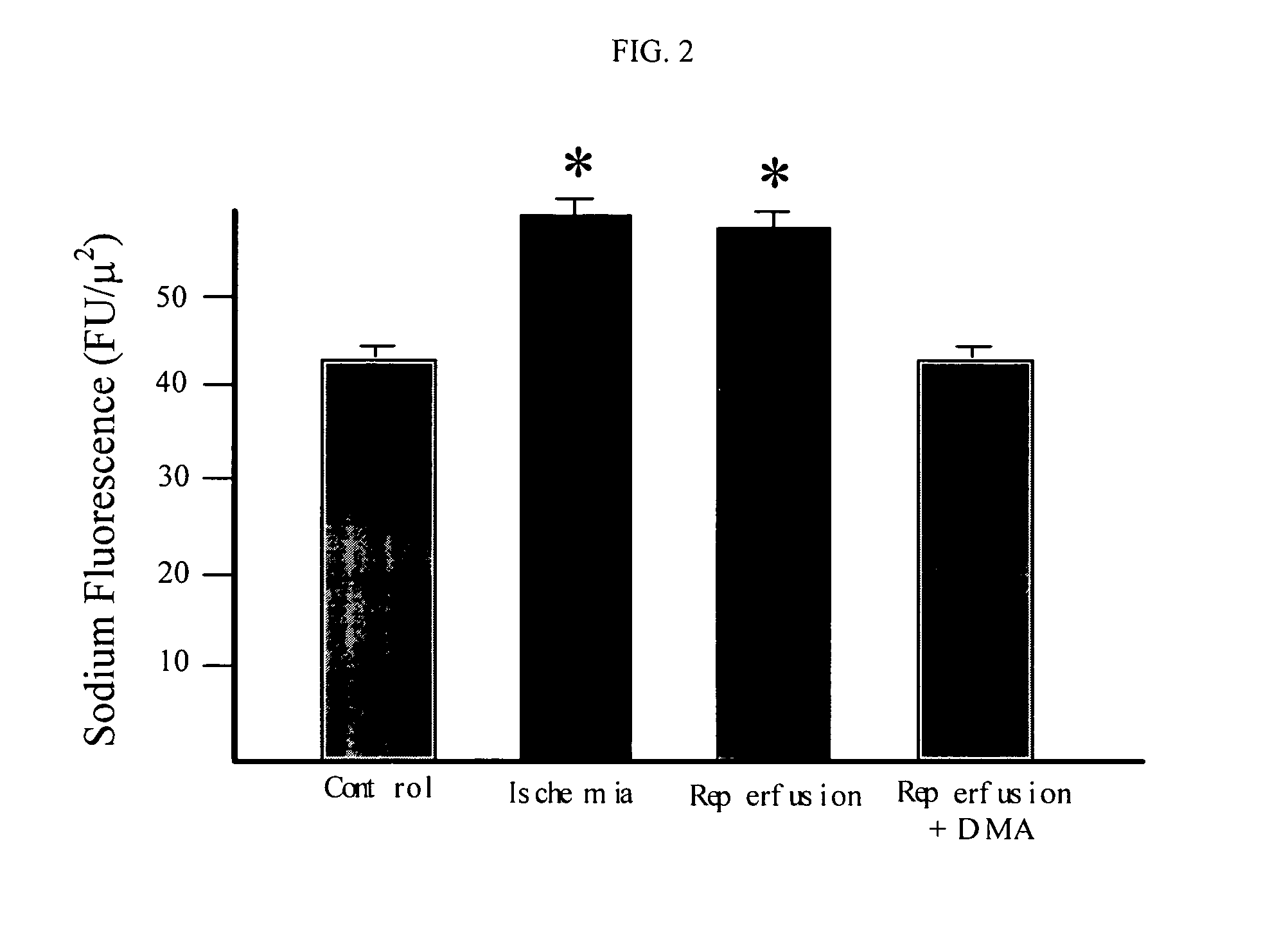
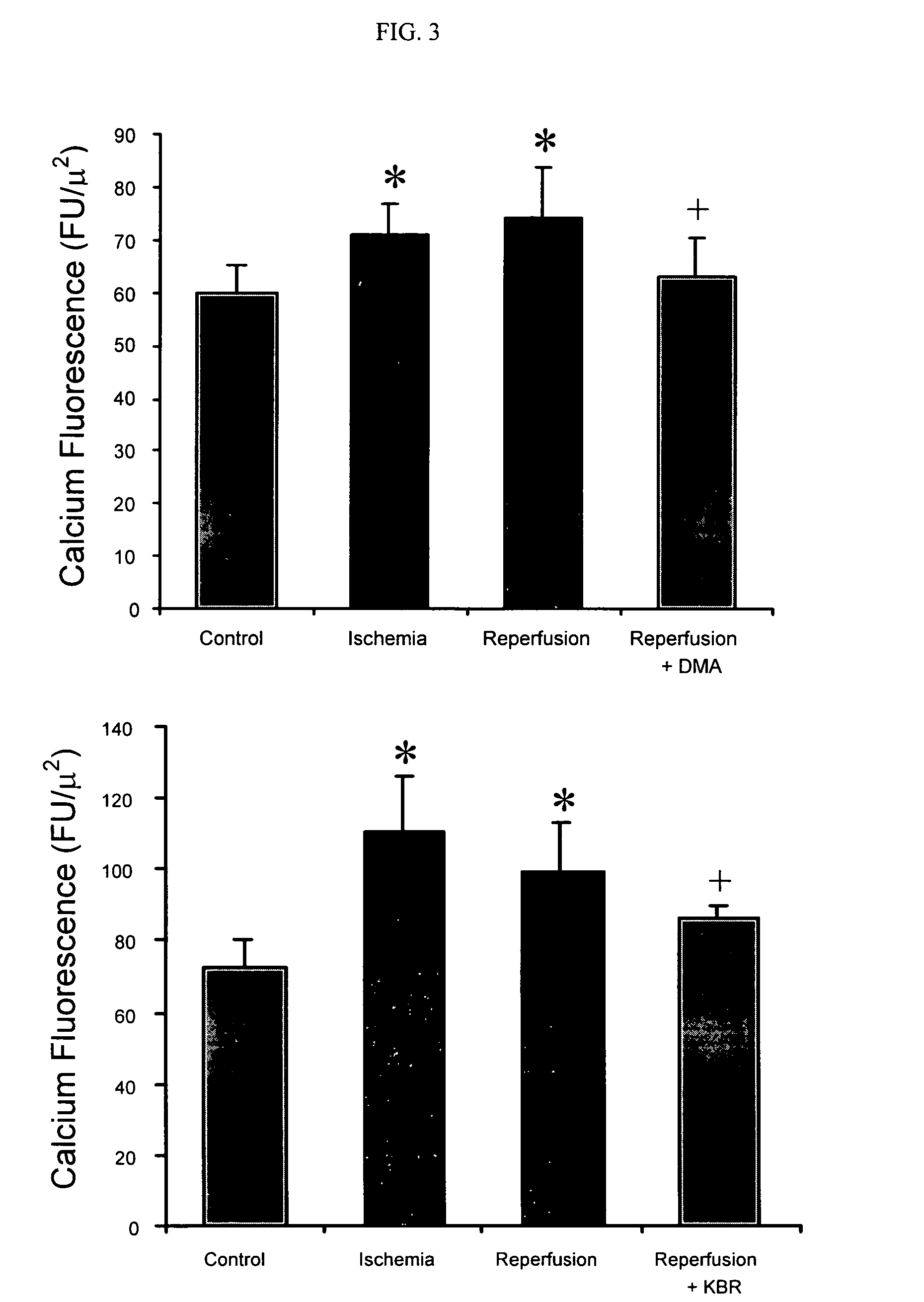
Cytoprotective thereapeutic agents for the prevention of reperfusion injury following ischemic stroke
ActiveUS20070293436A1Reduce decreaseAvoid reverse motionBiocideNervous disorderReperfusion injuryCysteine thiolate
The present invention relates generally to the use of γ-glutamyl antioxidants, particularly γ-glutamyl-cysteine, as cytoprotective agents to prevent reperfusion injury (i.e., hemorrhagic transformation) of the blood-brain barrier during reperfusion following an ischemic stroke. The γ-glutamyl antioxidants can be used alone or used in combination with an agent which inhibits the reverse movement of Na / Ca exchange in the blood-brain barrier such as 2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea methanesulphonate (KB-R7943).
Owner:ROSALIND FRANKLIN UNIVERSITY OF MEDICINE AND SCIENCE
Method and kit for imaging and treating organs and tissues
InactiveUS20020052594A1High resolutionStrong specificityElectrotherapyMedical devicesAntibody fragmentsImaging agent
Provided are methods and compositions for detecting and treating normal, hypoplastic, ectopic or remnant tissue, organ or cells in a mammal. The method comprises parenterally injecting a mammalian subject, at a locus and by a route providing access to said tissue or organ, with an composition comprising antibody / fragment which specifically binds to targeted organ, tissue or cell. The antibody / fragment may be administered alone, or labeled or conjugated with an imaging, therapeutic, cytoprotective or activating agent.
Owner:IMMUNOMEDICS INC
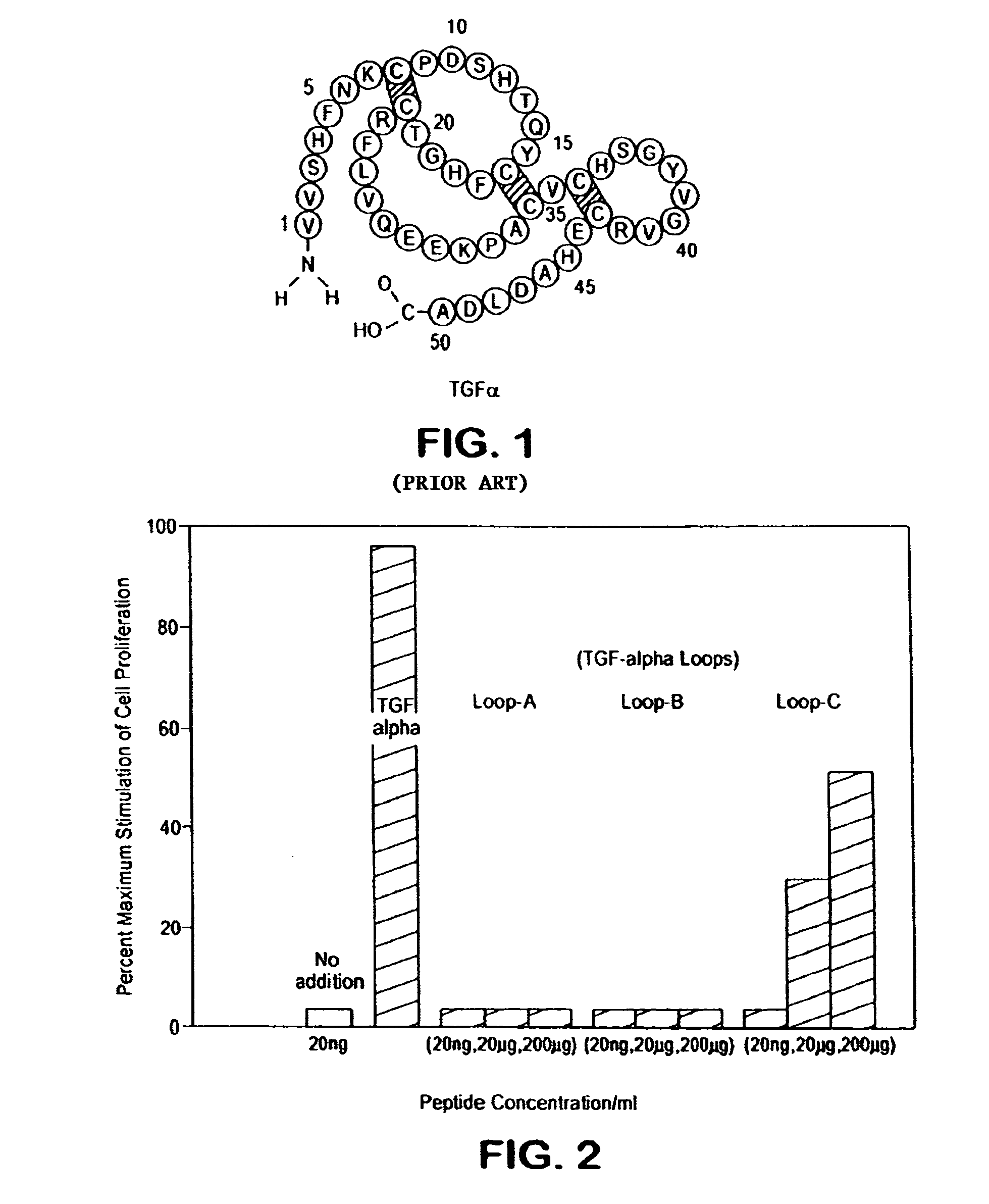
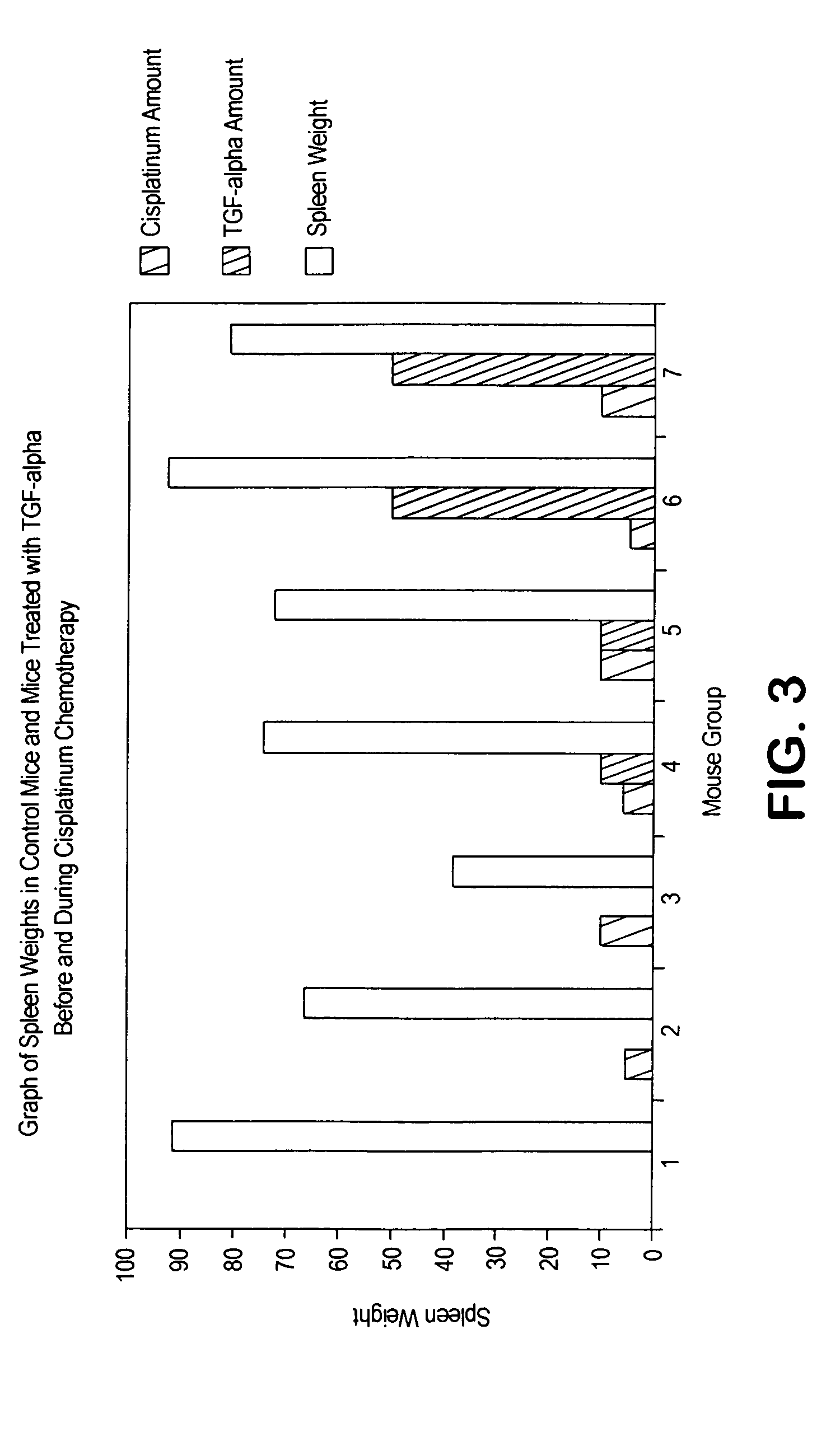
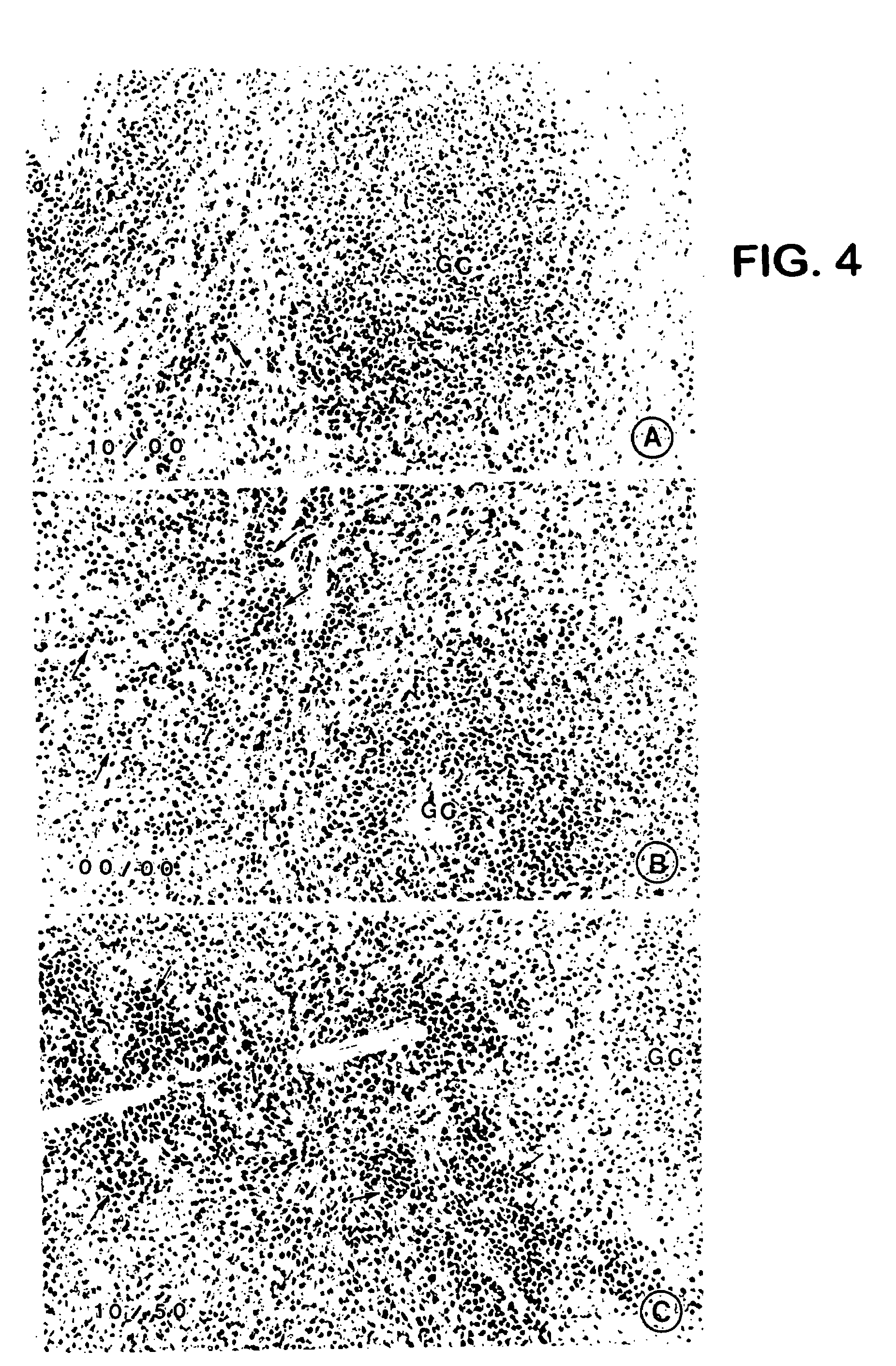
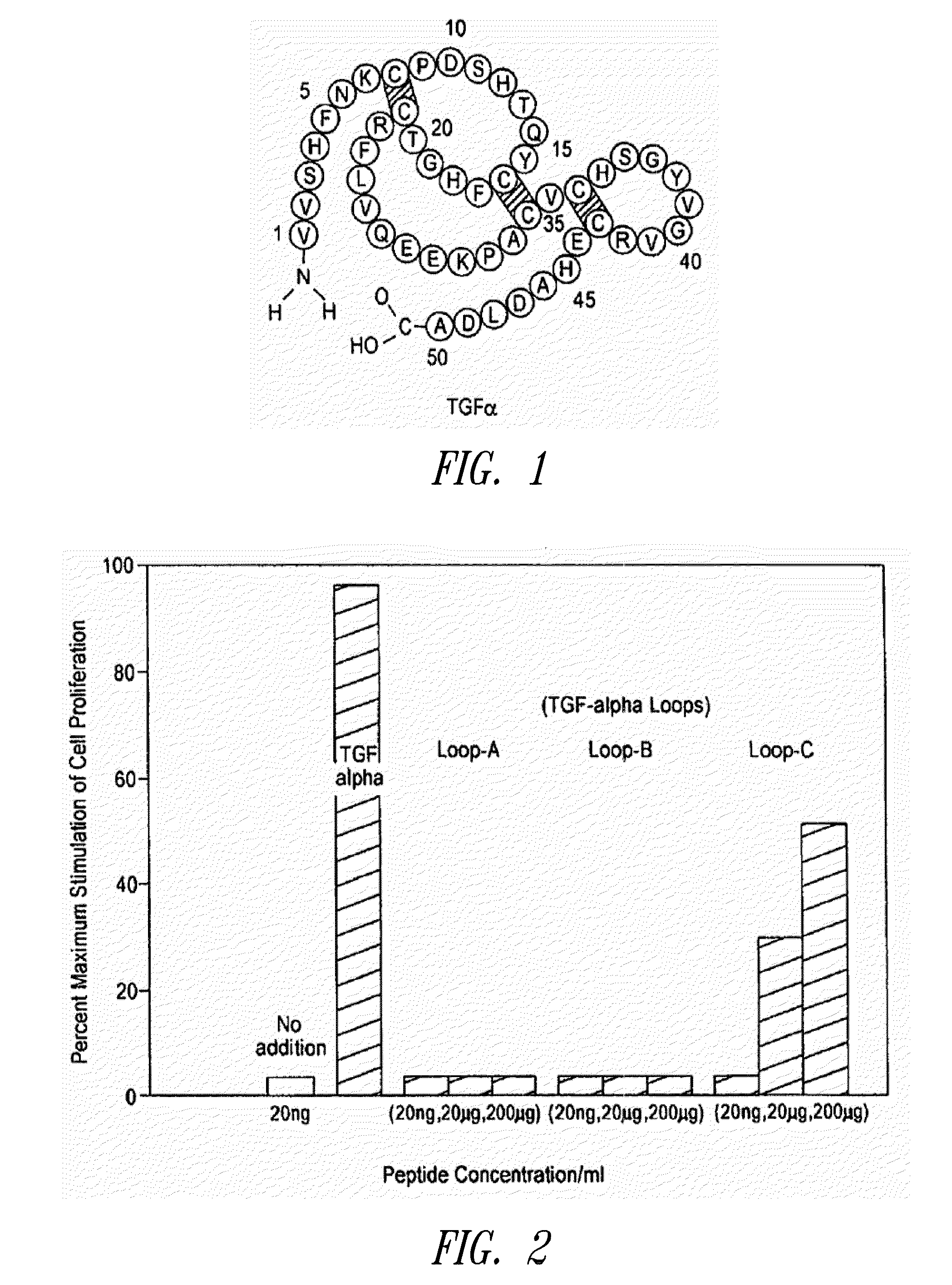
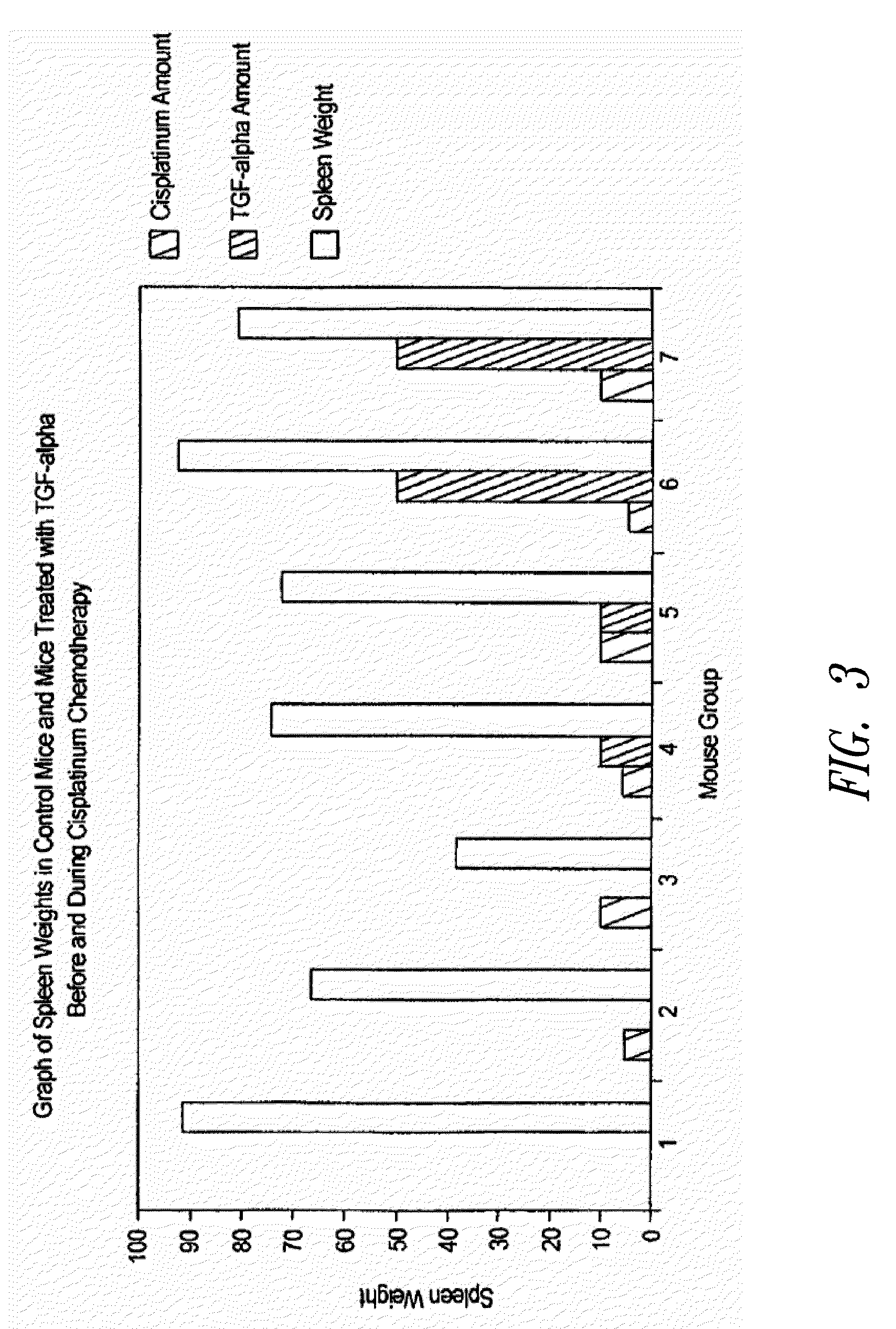
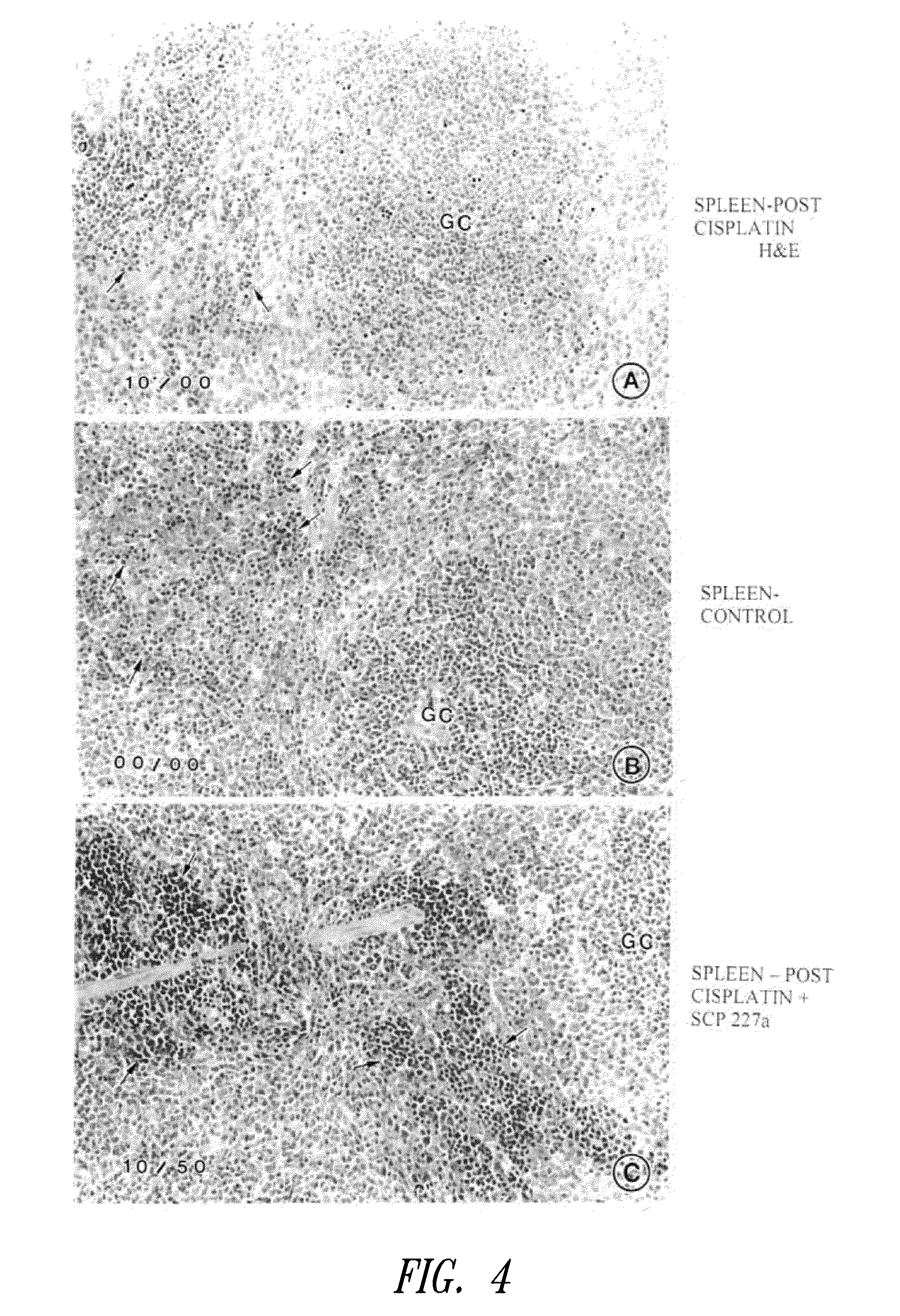
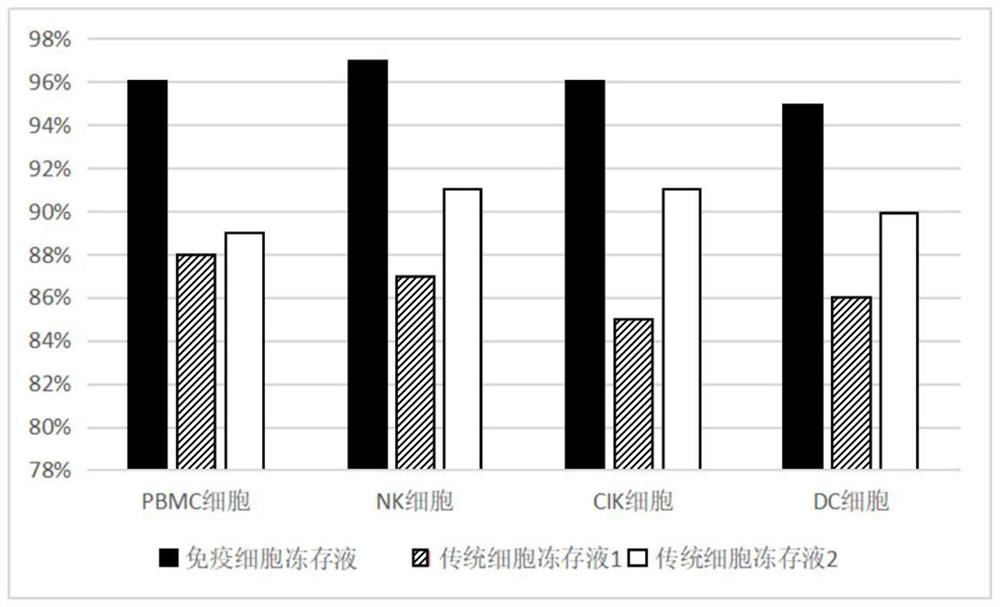
Loop peptide and TGFalpha for stimulating stem cell proliferation and migration
InactiveUS7365172B2Stimulate hematopoiesisAntibacterial agentsOrganic active ingredientsSide effectCytotoxicity
Owner:APPLIED PROTEIN SCI LLC
Aryl and heteroaryl propene amides, derivatives thereof and therapeutic uses thereof
Compounds useful as antiproliferative agents, radioprotective agents and cytoprotective agents, including, for example, anticancer agents, are provided according to formula I: wherein ring A, ring B,,X, R1, R2, R3, a, and b are as defined herein.
Owner:TEMPLE UNIVERSITY
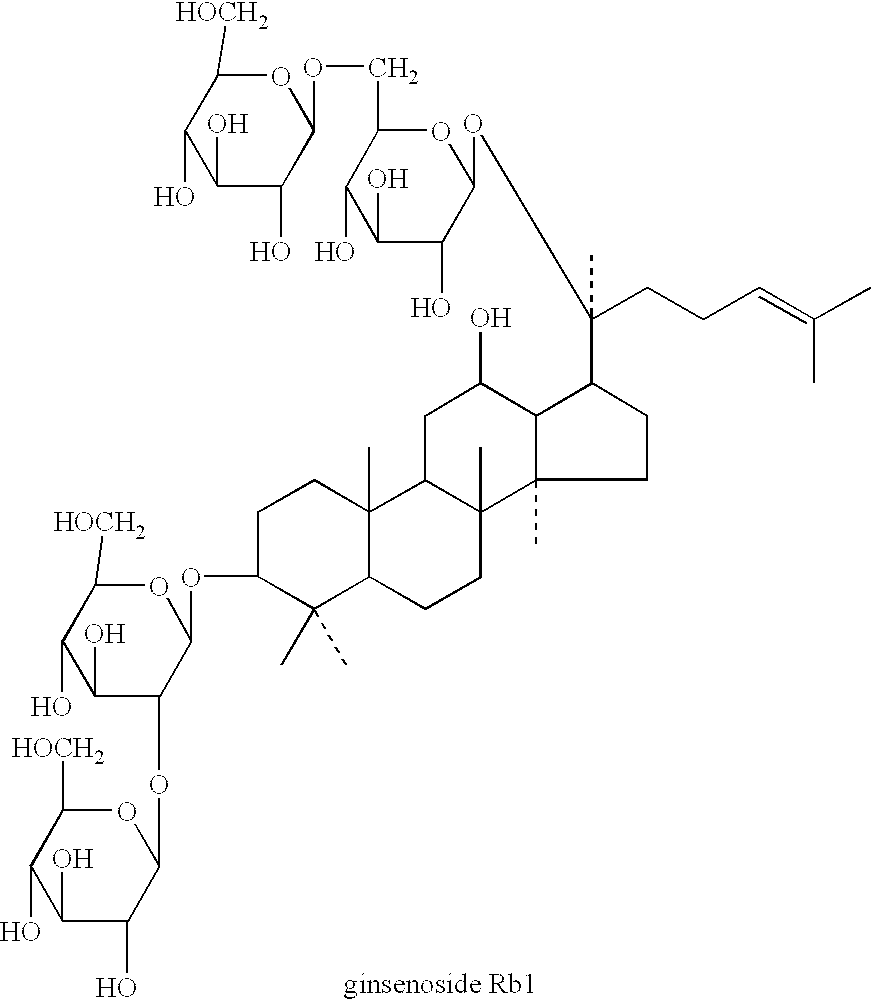
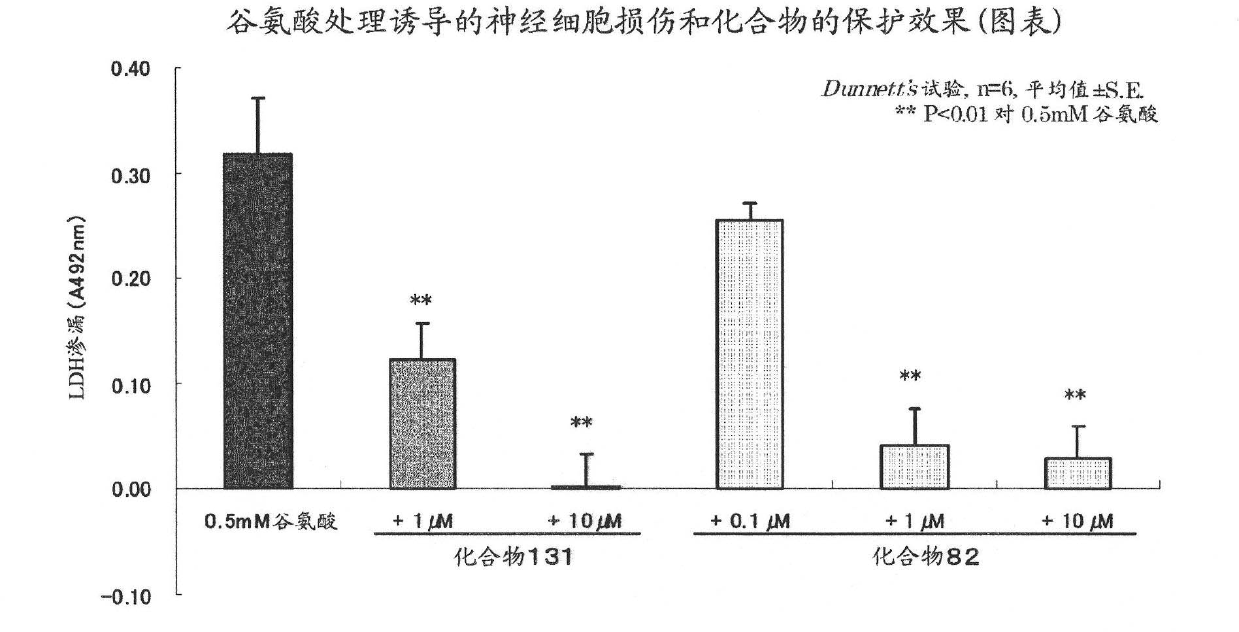
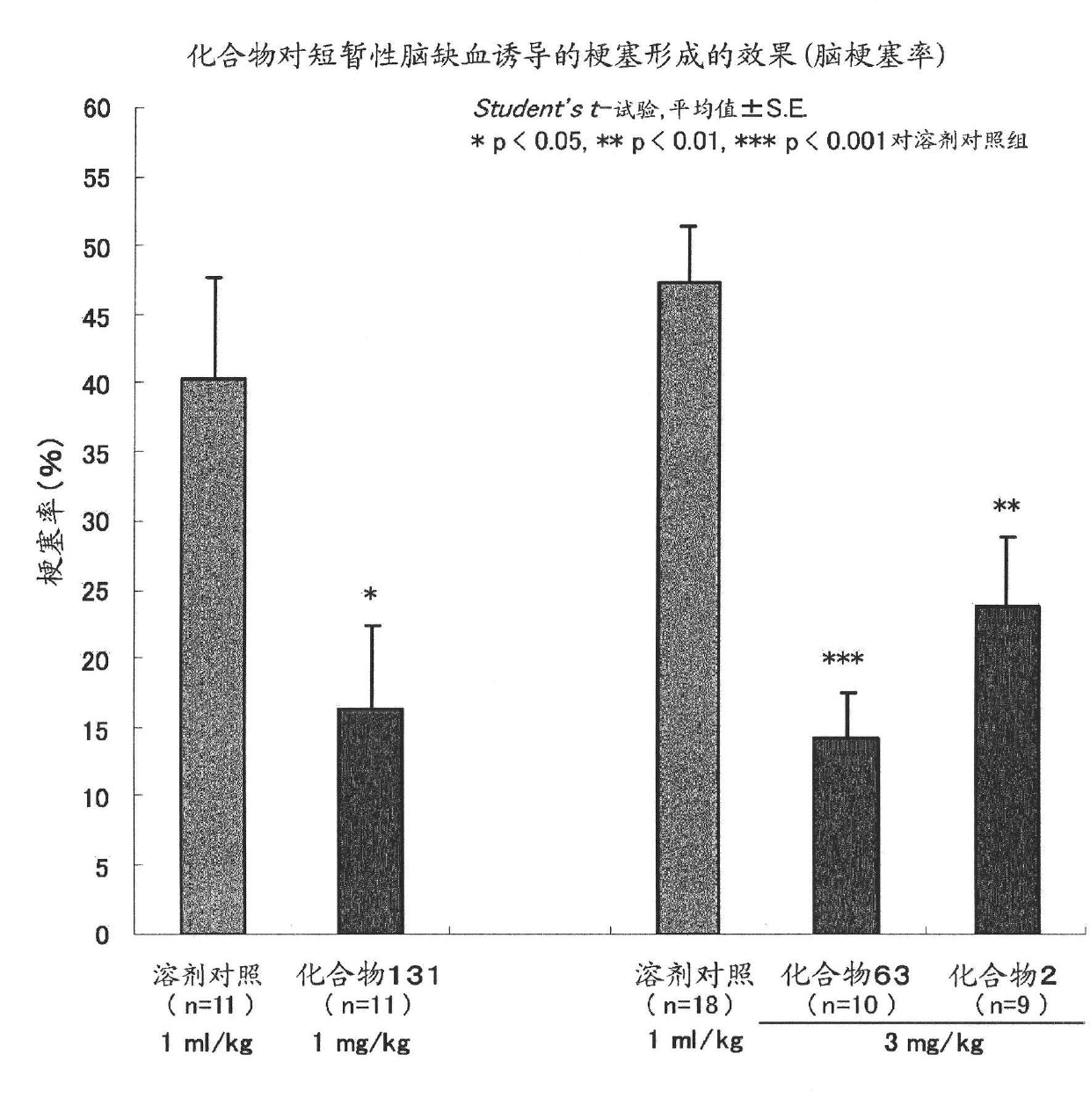
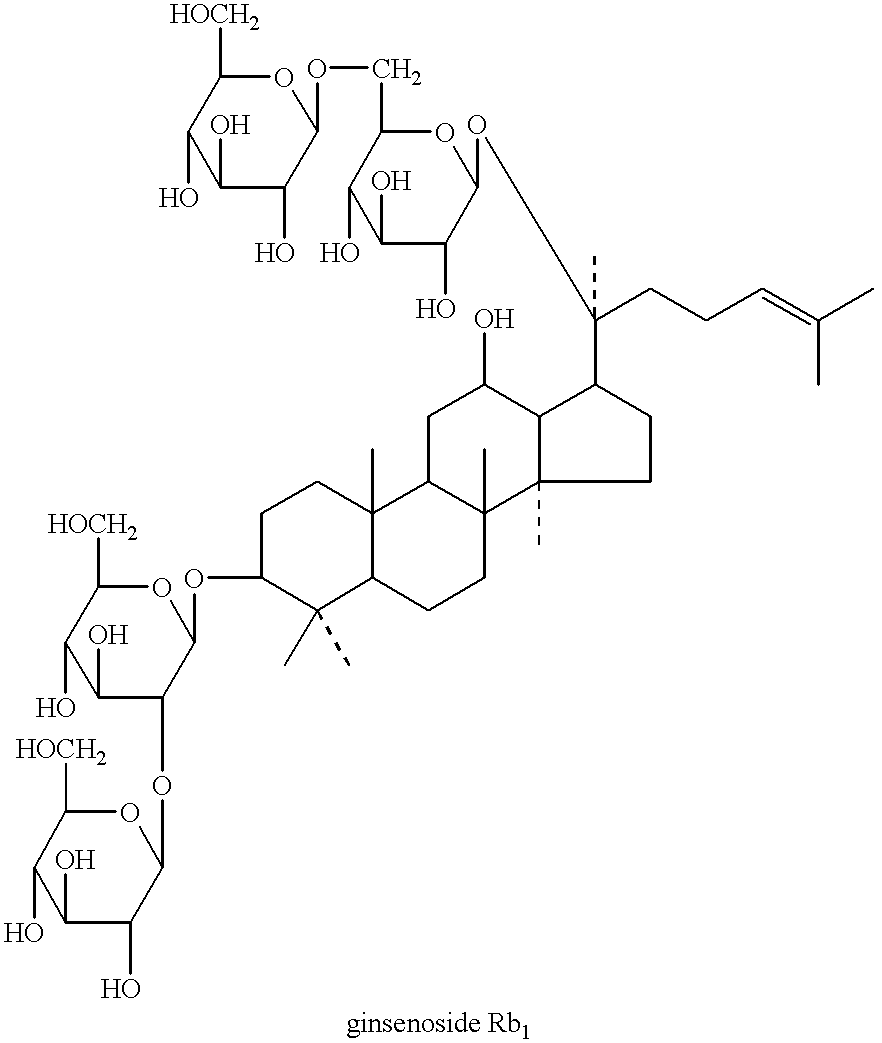
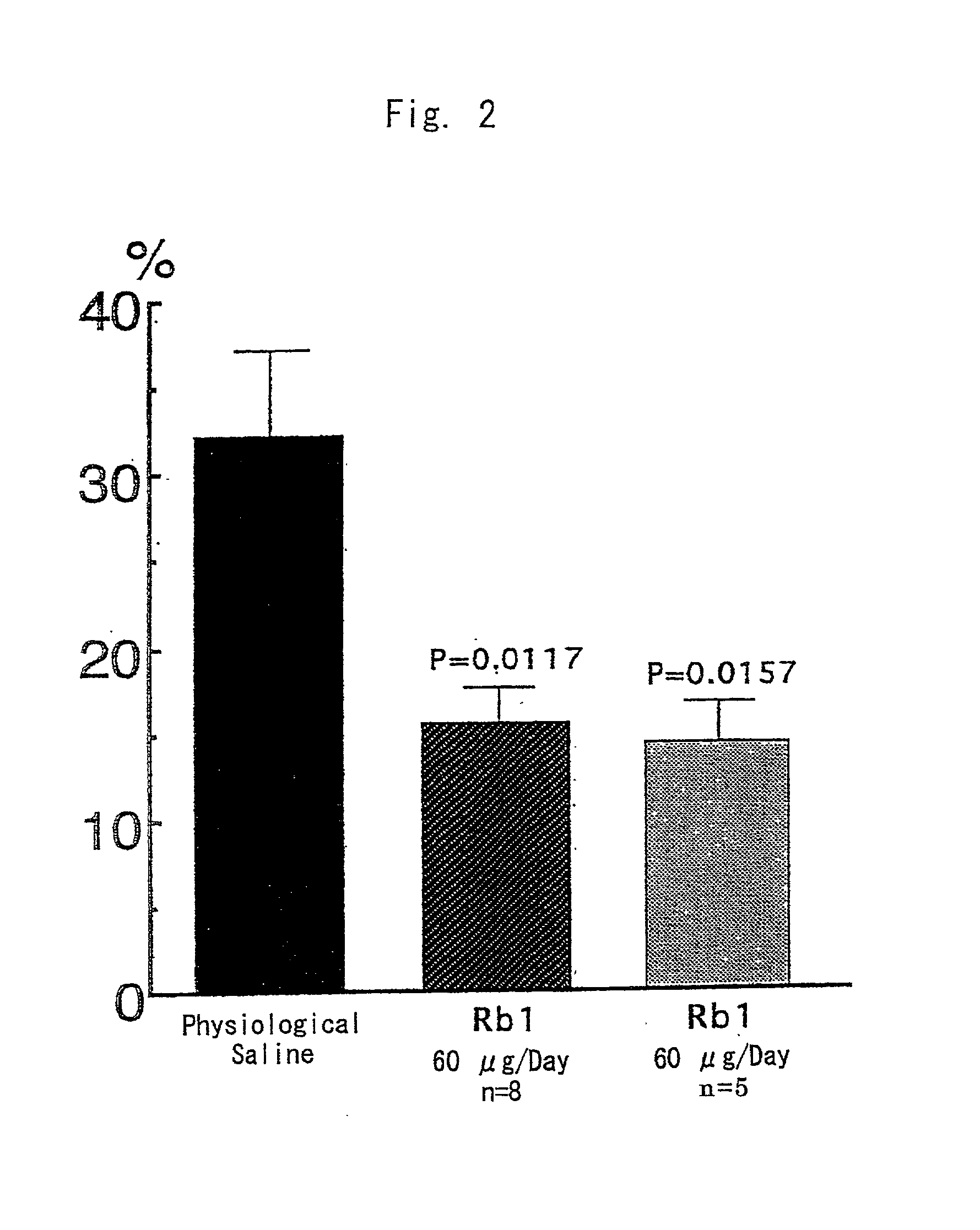
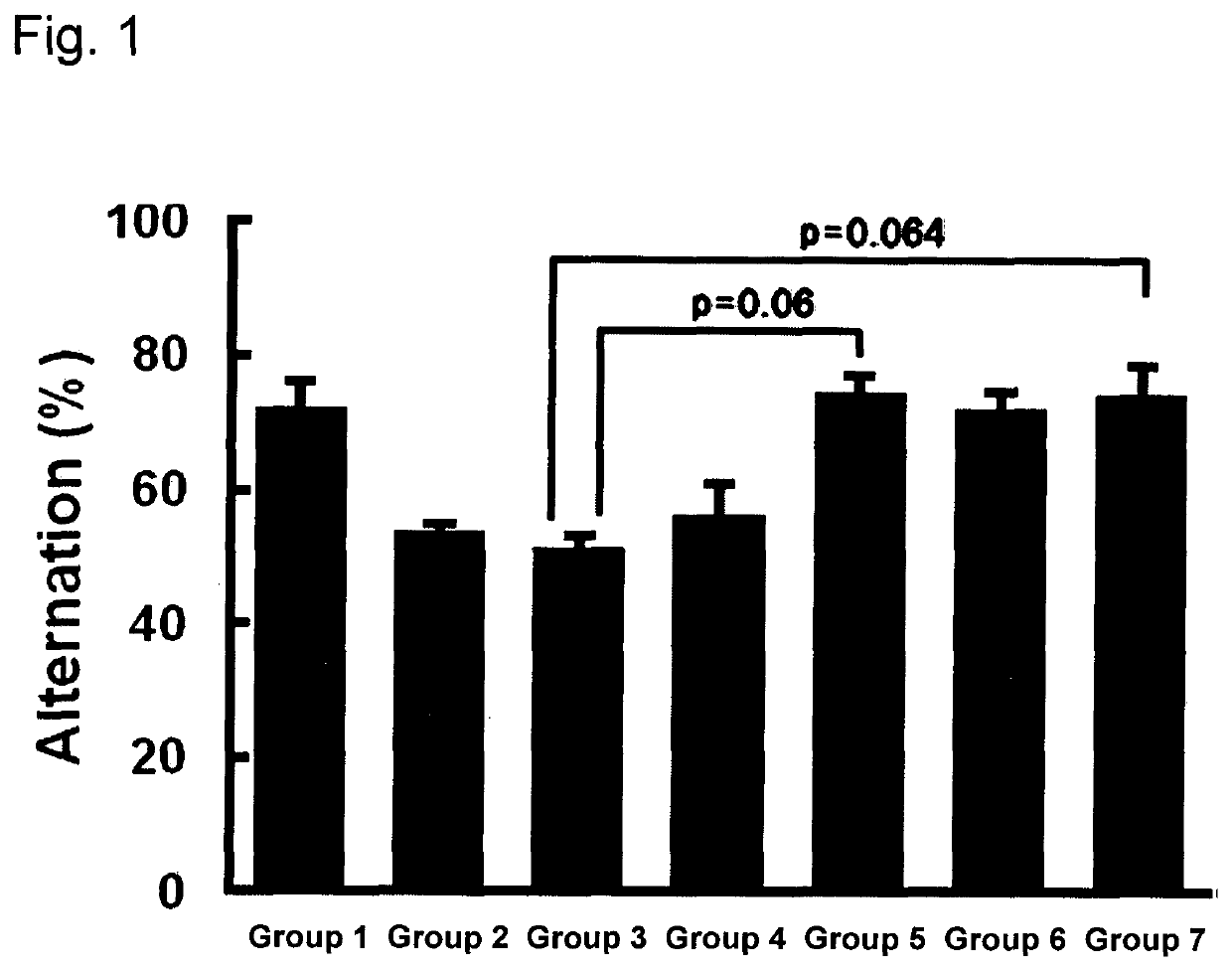
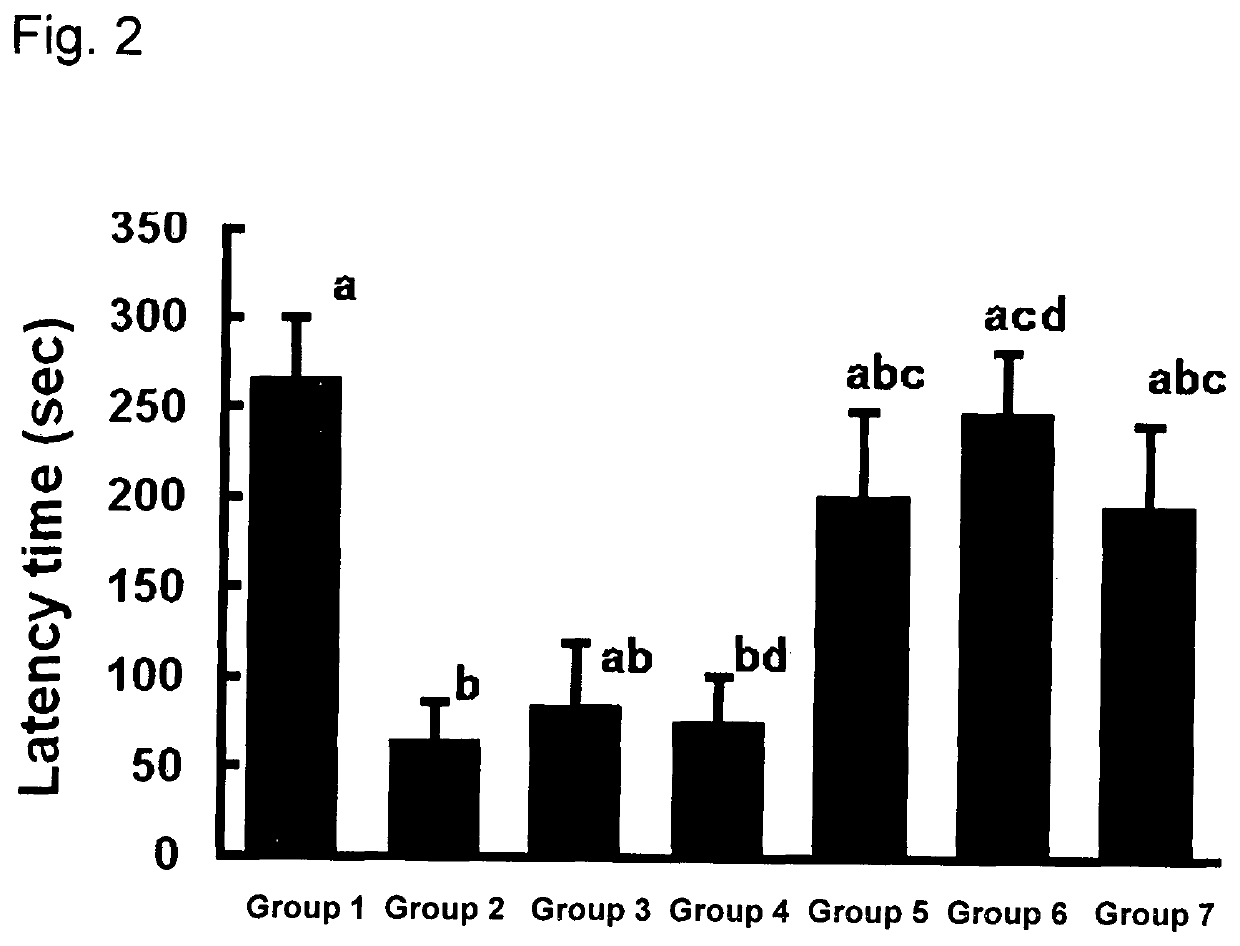
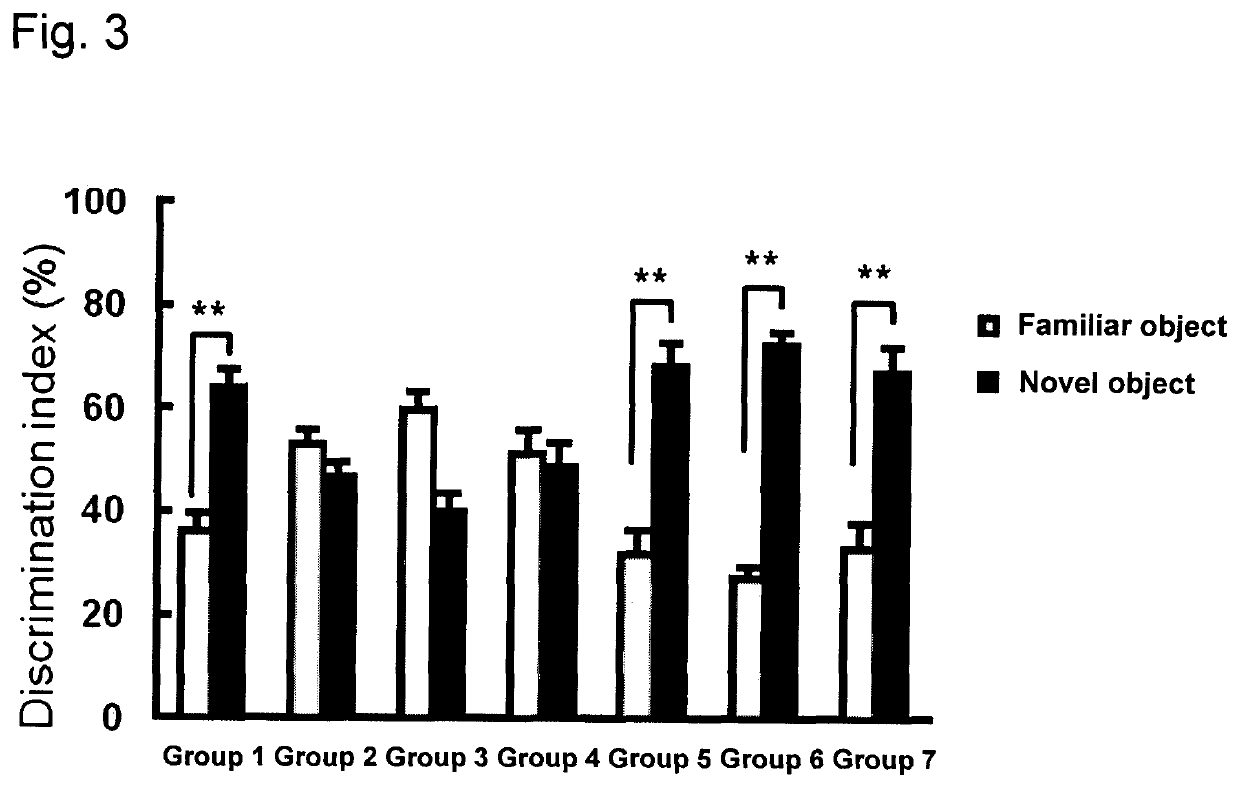
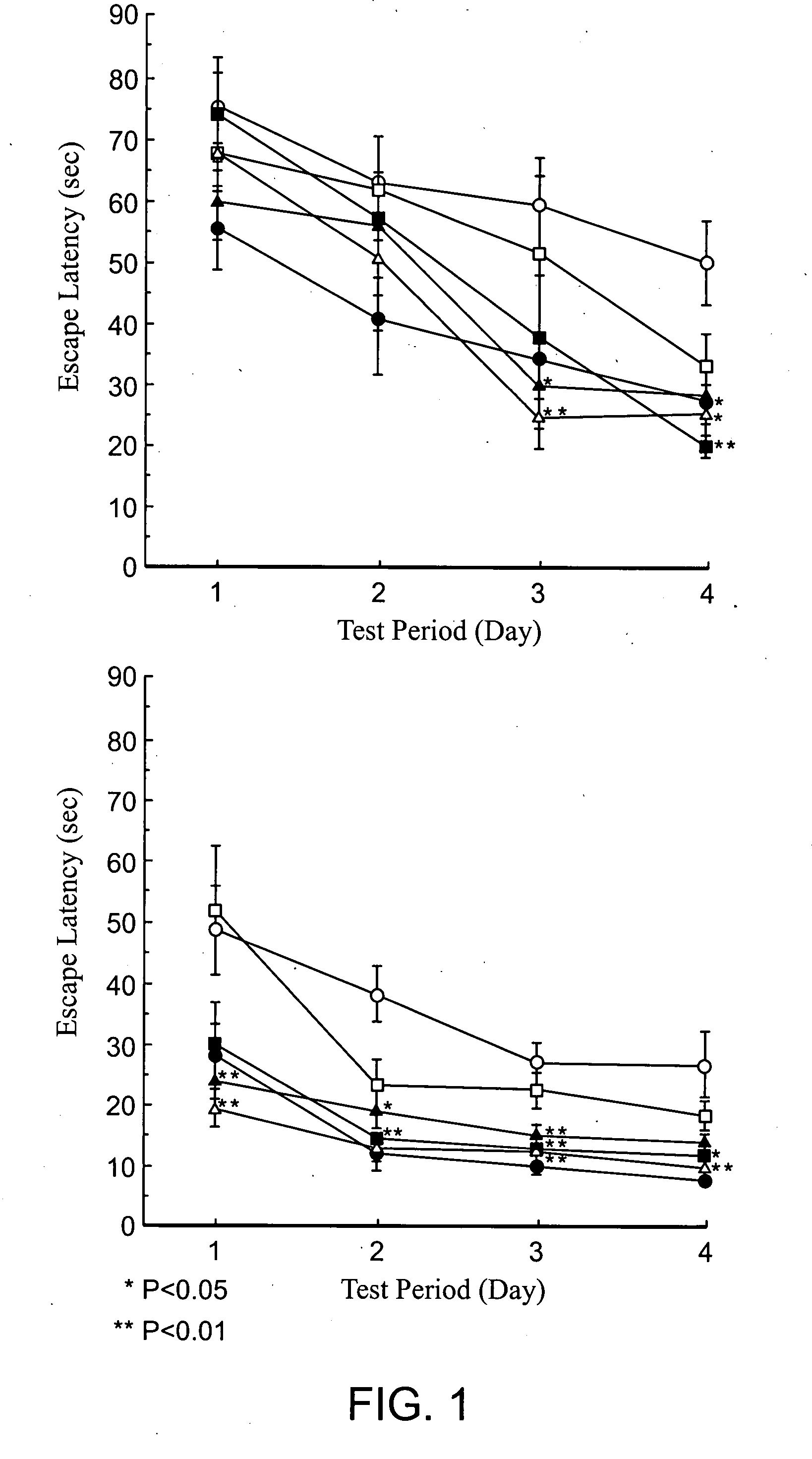
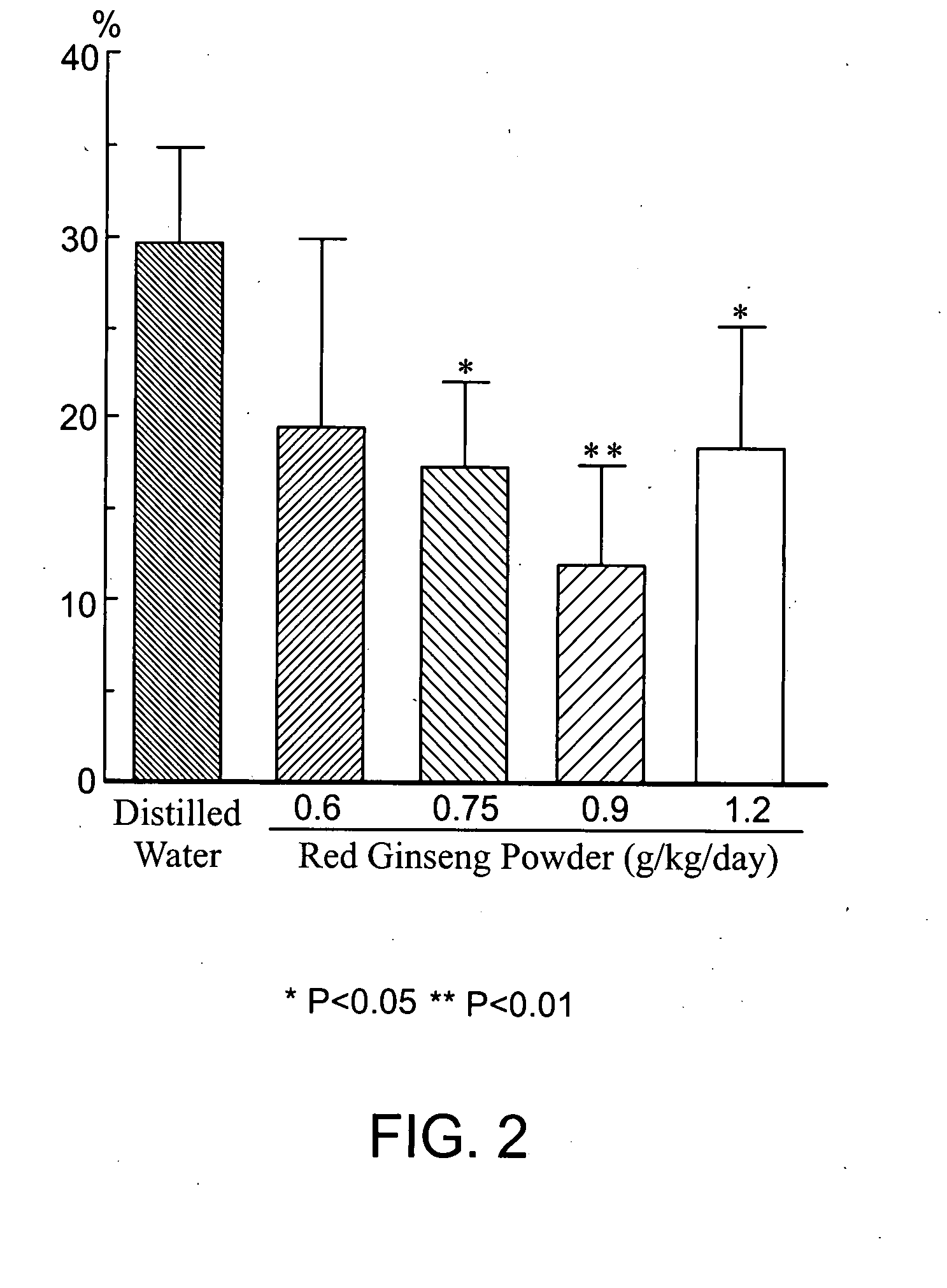
Brain cell or nerve cell-protective agents comprising ginsenoside Rb1
The present invention provides preparations for efficaciously administering ginsenoside Rb1 or its salt useful as cytoprotective agents. More particularly, the present invention provides pharmaceutical compositions comprising ginsenoside Rb1 or its salt for inhibiting apoptosis or apoptosis-like cell death or pharmaceutical compositions comprising ginsenoside Rb1 or its salt for promoting the expression of a cell death-inhibitory gene product Bcl-xL. Further, the present invention provides preparations for intravenous administration comprising ginsenoside Rb1 or its salt. The above pharmaceutical compositions contain ginsenoside Rb1 or its salt at low extracellular concentrations in lesion, preferably at 1 ng / ml or less and still preferably at 1 to 100 fg / ml. These compositions promote the expression of the cell death-inhibitory gene product Bcl-xL and inhibit apoptosis or apoptosis-like cell death. The above preparations for intravenous administration are useful for therapy, prevention or treatment of many diseases, in particular, brain and nervous diseases.
Owner:JAPAN SCI & TECH CORP
Excrement preserving fluid and preparation method thereof
InactiveCN111183974AImpaired inhibitionLong storage timeDead animal preservationBiotechnologyMicroorganism
The invention aims to provide an excrement preserving fluid which is applied to excrement preservation. The excrement preserving fluid can preserve an excrement sample for a long time at normal temperature, can effectively inhibit DNA degradation and cell damage in the excrement sample, maintains cell morphology, and has a good inhibiting effect on the activity of degradation enzymes and microbes.The invention also provides a preparation method of the excrement preserving fluid. The excrement preserving fluid prepared by the preparation method is used for preserving excrement. The excrement preserving fluid comprises 10-500 mmol / L of a chelating agent, 1-100 g / L of a bacteriostatic agent, 1-10 g / L of an ionic strength maintaining agent, 1-50% (v / v) of a cell protective agent and 1-50 g / Lof a denaturing agent.
Owner:广州维帝医疗技术有限公司
Nano-compound and its preparation method and application
ActiveCN108261548AThe antitumor effect has no effect onAnti-tumor effectsOrganic active ingredientsPharmaceutical non-active ingredientsCytoplasmWilms' tumor
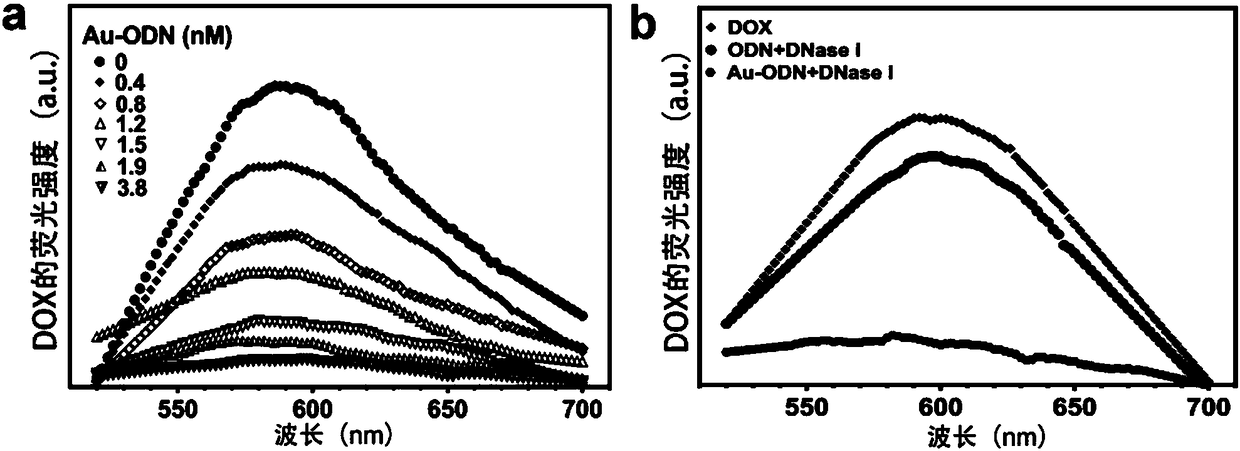
The invention discloses a nano-compound and its preparation method and application in preparation of a chemotherapeutic drug cytoprotective agent and belongs to the technical field of biotechnology and biomedical materials. The nano-compound comprises a GC base pair-rich oligonucleotide and a gold nanocage. The end 3' of one chain in oligonucleotide is connected to the gold nanocage through a thiol group. The content of the GC base pairs in the oligonucleotide is greater than 50%. The nano-compound Au-ODN can enter cytoplasm through endocytosis, can capture the chemotherapeutic drug entering the cell, and can inhibit the effect of the drug entering the nucleus. 70-80% of the nano-compound can target liver tissue, and only about 5% of the nano-compound can enter cancer tissue. Therefore, the nano-compound Au-ODN can be used for preparing a chemotherapeutic drug cytoprotective agent for preventing liver cells from being damaged by drugs and has no influence on the anti-tumor effect of the chemotherapy drugs.
Owner:ZHEJIANG UNIV
LOOP PEPTIDE AND TGF alpha FOR STIMULATING STEM CELL PROLIFERATION AND MIGRATION
InactiveUS20090068139A1Stimulate hematopoiesisSenses disorderNervous disorderSide effectCytotoxicity
There is disclosed a novel genus of small peptides, smaller than human TGFα, identified as having TGFα biological activity and therefore being useful as pharmacologic agents. It is further disclosed that both TGFα and the genus of small peptides disclosed herein have therapeutic activity to stimulate hematopoiesis, e.g., in patients undergoing cytotoxic cancer chemotherapy, and also act as cytoprotective agents to protect patients undergoing cancer cytotoxic therapy from gastrointestinal (GI) side effects, such as mucositis, and otherwise to support the barrier function of the GI tract, such as when it is harmed by cytotoxic therapy.
Owner:TWARDZIK DANIEL R +2
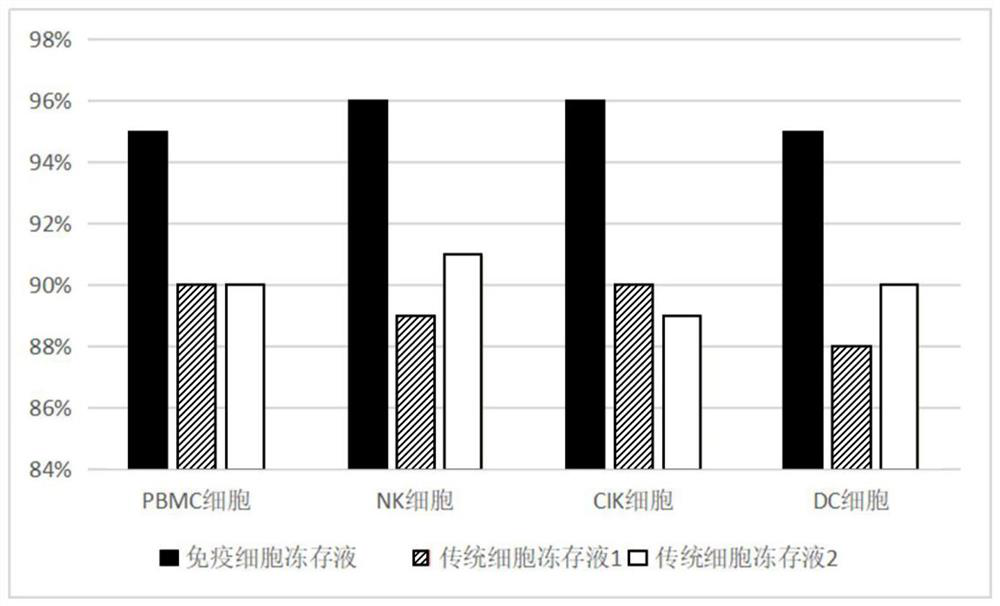
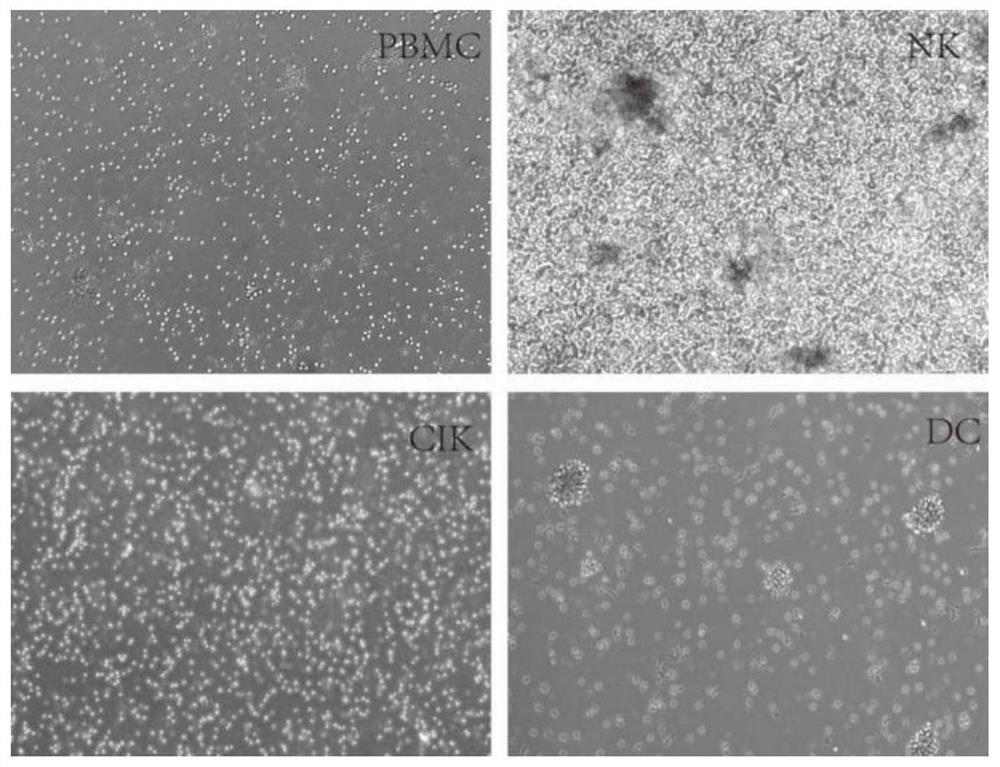
Immune cell freezing medium and preparation method and application thereof
PendingCN112806354AImprove cryopreservation recovery rateFree from damageDead animal preservationCryopreservationAlbumin
The invention provides an immune cell cryopreservation solution and a preparation method and application thereof. The immune cell cryopreservation solution is specifically prepared from human serum albumin, a cell balance solution, a cell stabilizer, a cell protective agent and an amino acid solution. The immune cell cryopreservation solution contains various cell protection components, so that internal and external injuries caused by ice crystals during cryopreservation of immune cells are avoided, and the recovery rate of the unfrozen immune cells is increased. The immune cell cryopreservation solution does not contain serum components, DMSO and the like, introduction of heterologous exogenous components and harmful components is avoided, and the immune cell cryopreservation solution is safer and more reliable in clinical application.
Owner:圣至润合(北京)生物科技有限公司
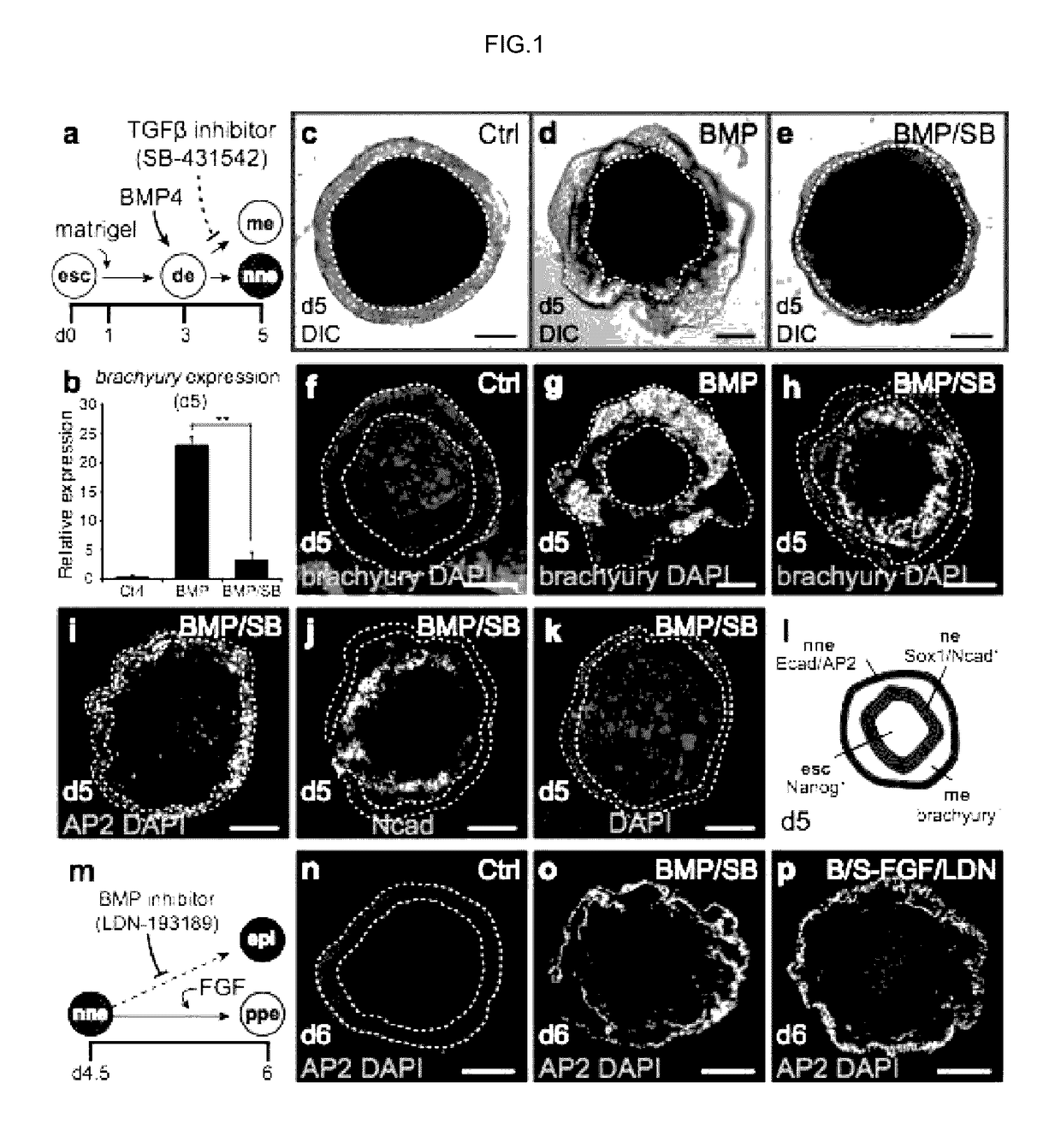
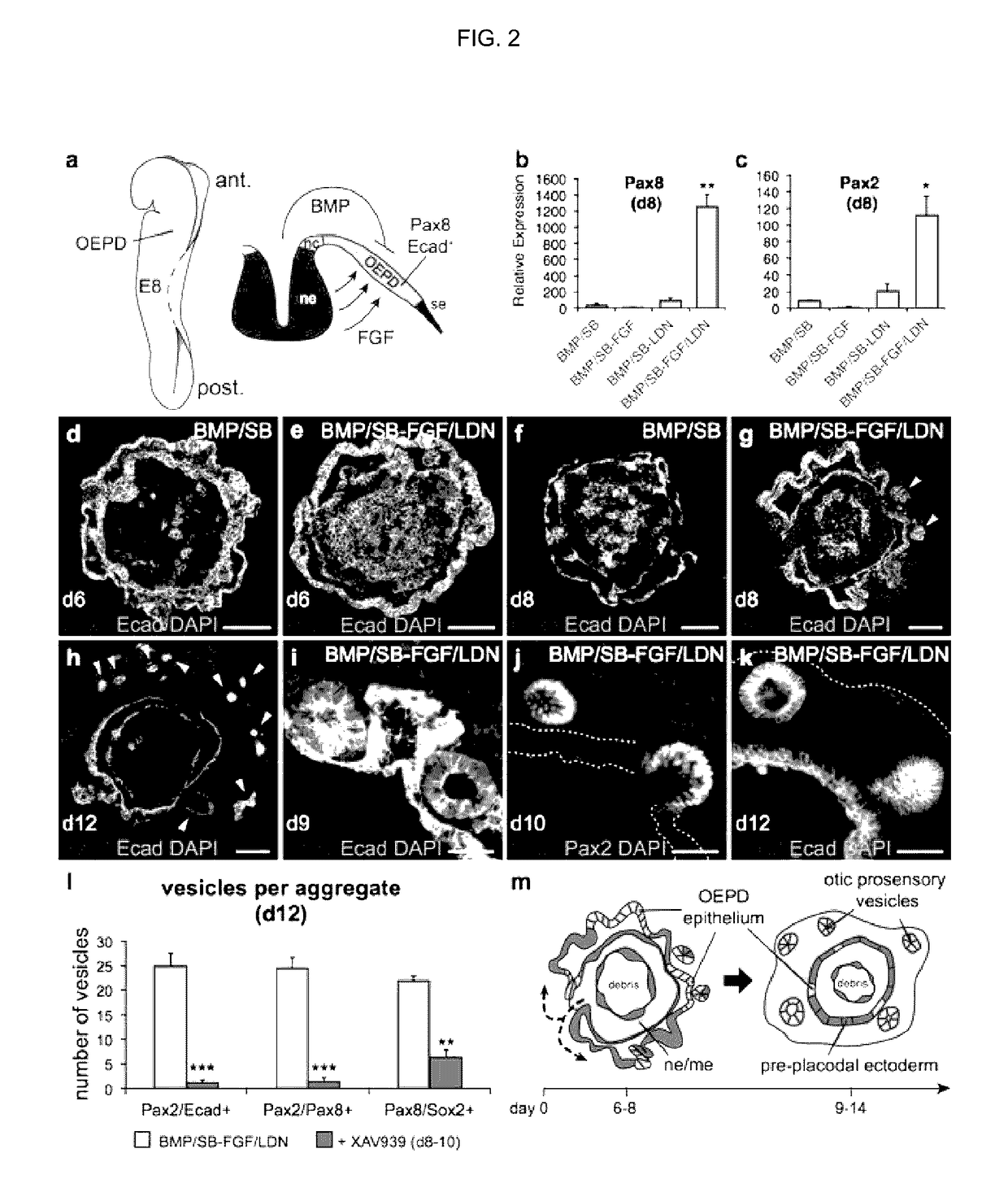
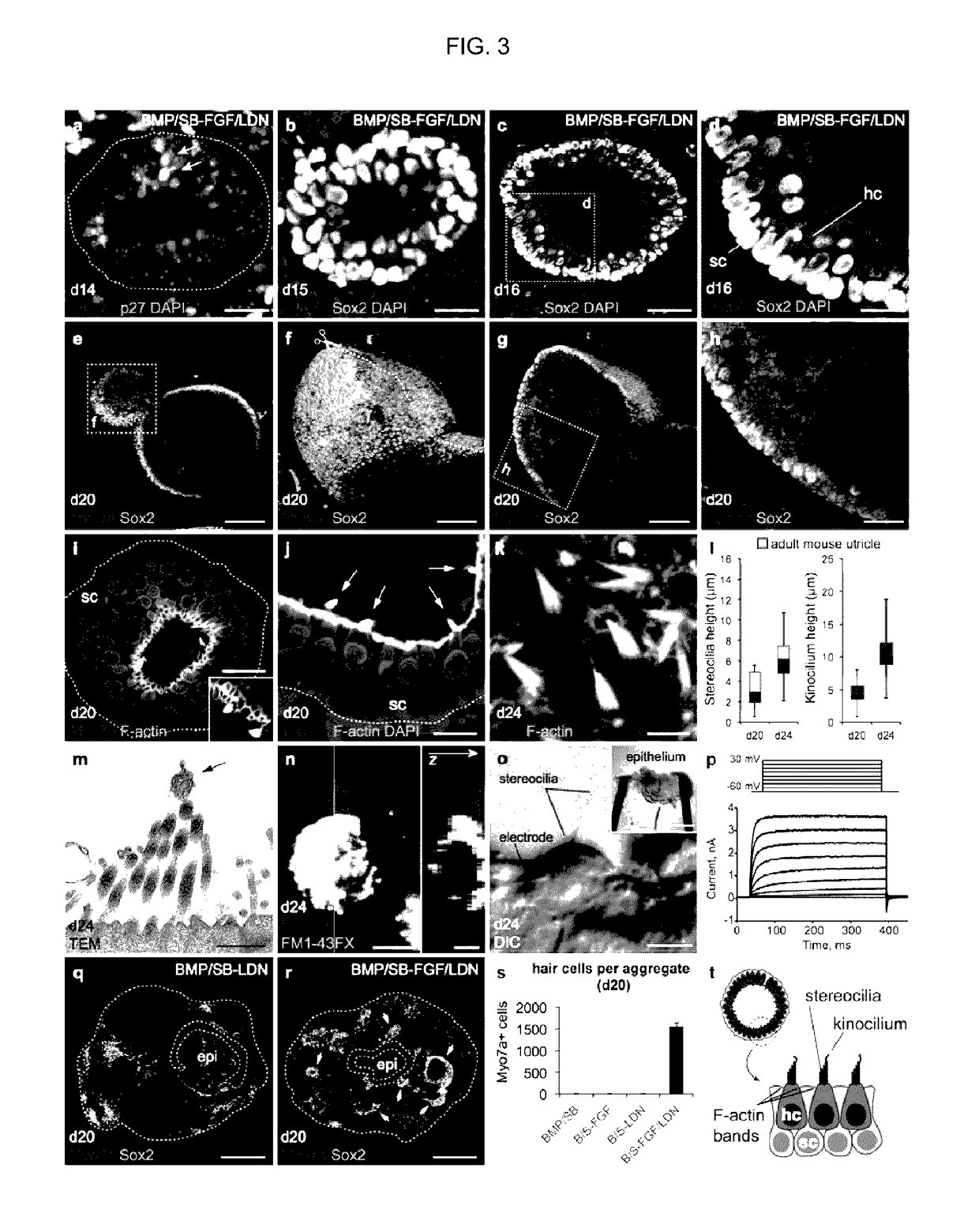
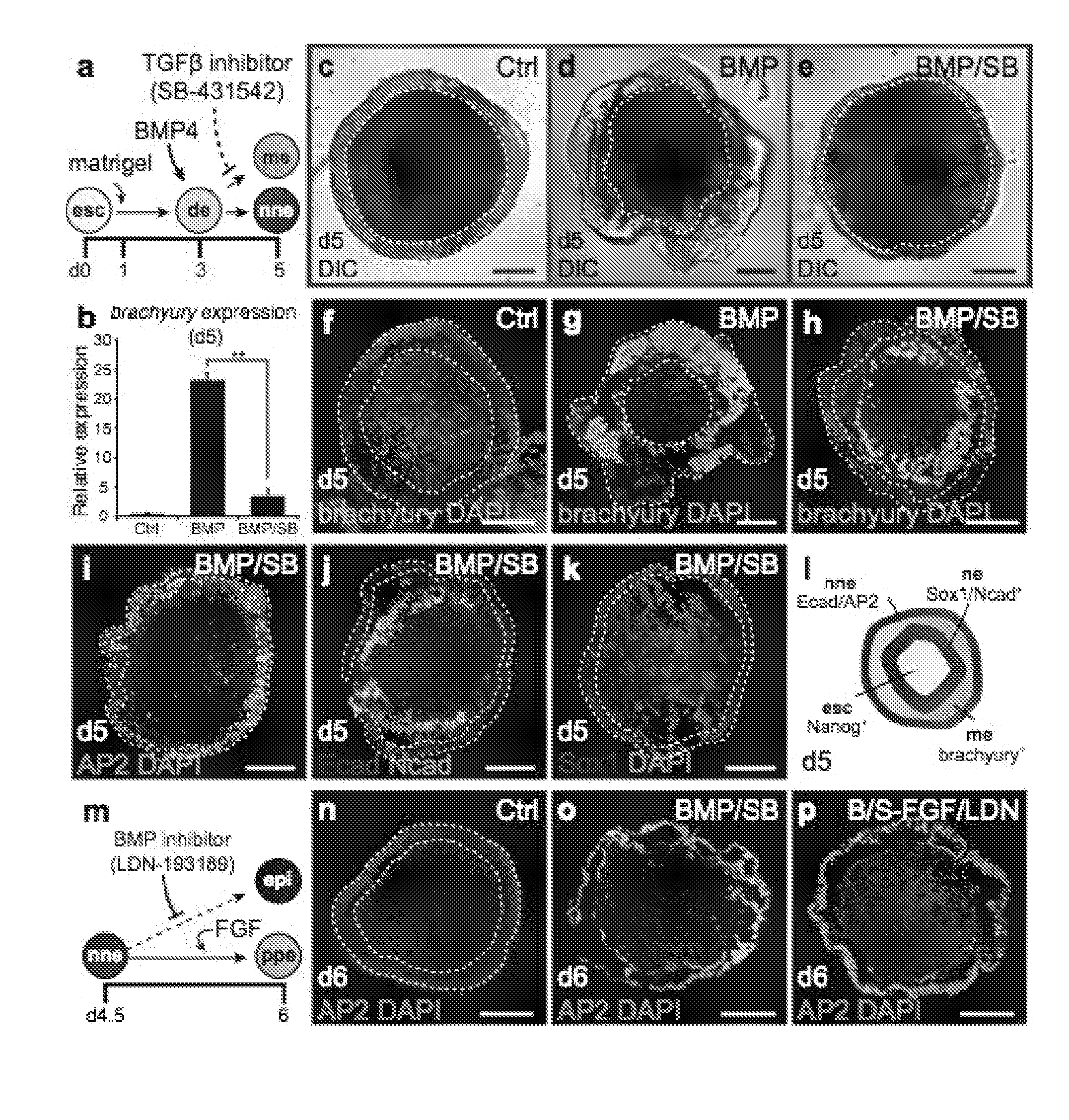
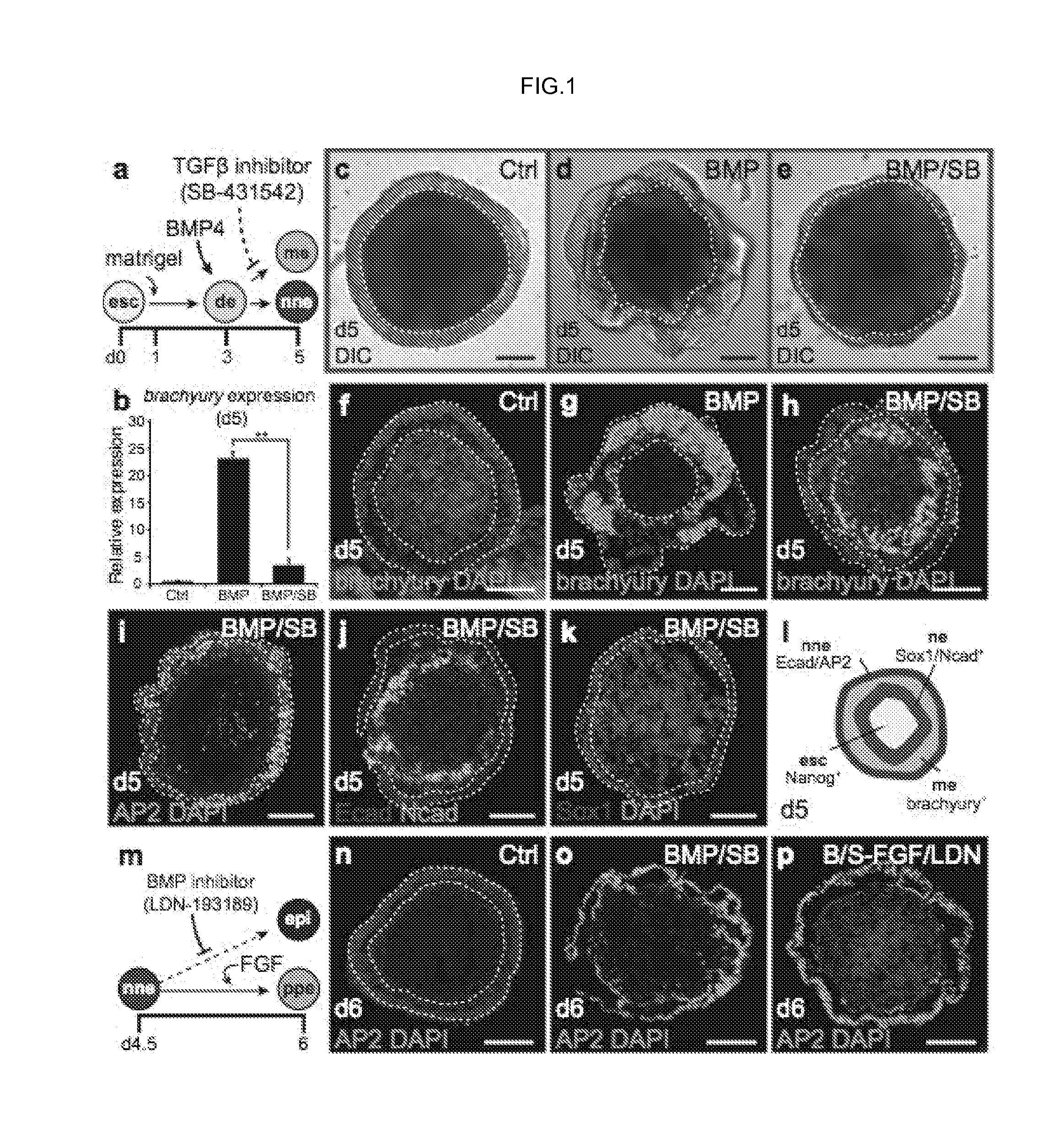
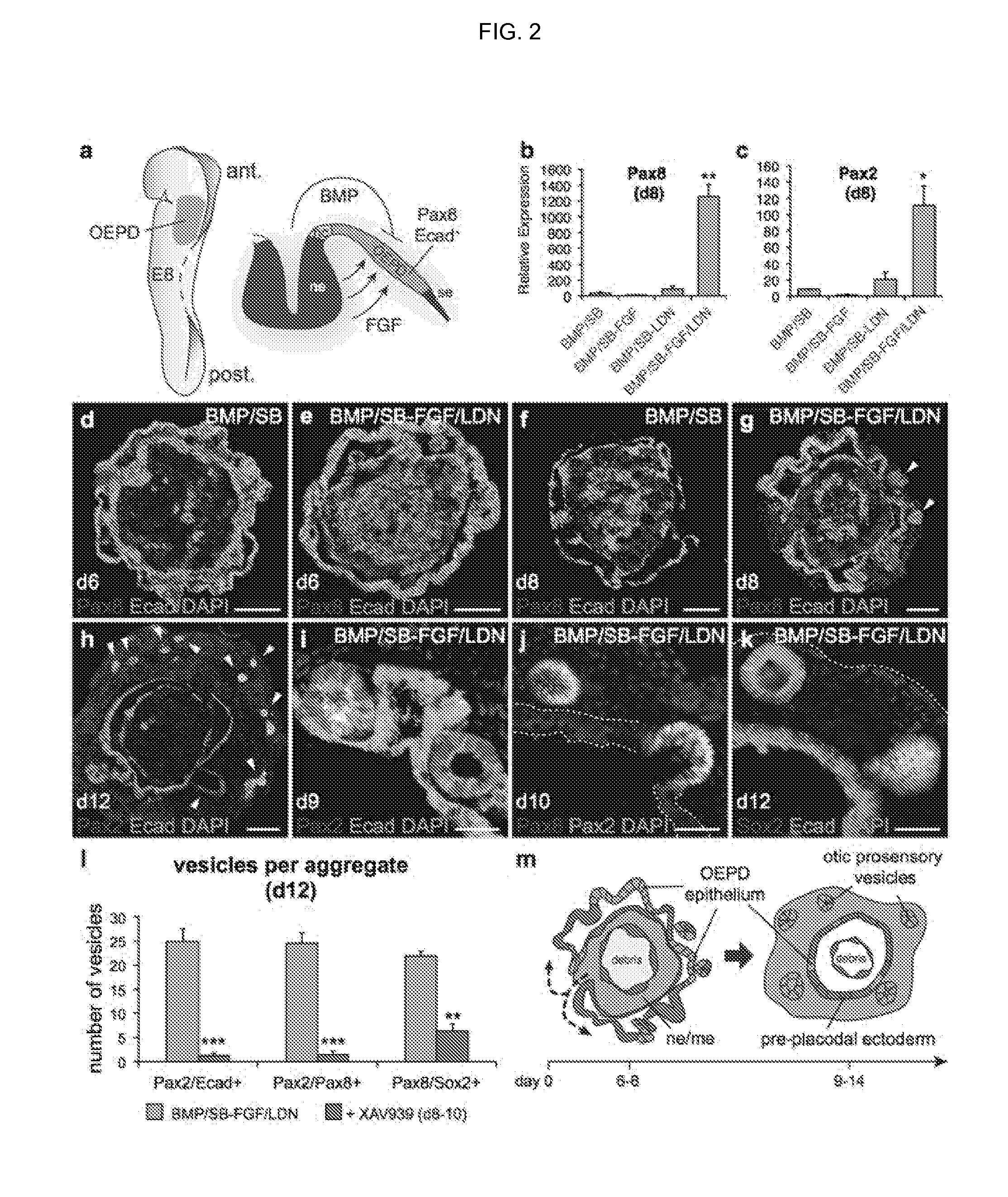
Methods for generating the inner ear and other cranial placode-derived tissues using pluripotent stem cells
ActiveUS9624468B2Epidermal cells/skin cellsNervous system cellsHair cell differentiationSignalling pathways
Disclosed herein are methods and compositions for generating cultures and isolated cell populations containing preplacodal ectoderms cells, otic placode cells, and inner ear sensory hair cells derived from pluripotent cells by modulating TGFβ, BMP, and FGF signaling pathways under defined culture conditions. Also described are methods for obtaining non-otic placodal tissues from pluripotent stem cells. Methods for identifying agents that induce or enhance differentiation and generation of hair cells are also disclosed. Methods for identifying cytoprotective agents for hair cells are also described.
Owner:INDIANA UNIV RES & TECH CORP
Cell protective agent containing metallothionein
InactiveCN102634479AAvoid immunogenicityPeptide/protein ingredientsArtificial cell constructsDiseaseCritical illness
The invention discloses cell protective agent containing metallothionein, which aims to solve the problems that blood cells are damaged during blood perfusion, corresponding tissues and organs during growth are damaged after the cells are cultured and augmented in vitro and transplanted and survival rate is low and the like, and can be used for blood perfusion treatment method and cell treatment method including a method for protecting the corresponding tissues and organs after vitro-culturing, augmenting and transplanting of the cells. The cell protective containing metal sulfur protein can be widely used for protecting blood cells of human bodies with diseases such as uremia, critical illness, intoxication, systemic lupus erythematosus (SLE), severe hepatitis and the like during blood perfusion treatment, and protecting corresponding cultured and augmented cells or transplanted and hyperplasia cells of the corresponding tissues and organs in the cell treatment of diseases caused by severe damage or reduction of the cells.
Owner:汪志友
Microbe sample preservation solution for gene detection, preparation method and kit thereof, and application of kit
ActiveCN108546646AEffective inactivationEffective inactivation abilityMicroorganism preservationHigh concentrationGlycerol
The invention provides a microbe sample preservation solution for gene detection, a preparation method and a kit thereof, and an application of the kit, and belongs to the technical field of bioengineering. The microbe sample preservation solution for gene detection has effective and comprehensive microbe inactivation capability; and low-concentration ethanol is used to thoroughly inactivate bacteria in the storage process of a sample, and avoids inactivation failure caused by the stress reaction of bacteria due to high-concentration ethanol. The microbe sample preservation solution has good permeability on the premise of not destroying the cell structure, and effectively destroys the protein activity of bacteria and viruses and the activities of various nucleases to make the bacteria, viruses and nucleases not have of infection or proliferation ability, so the thorough inactivation effect is achieved. A glycerol-containing cytoprotective agent and a physiological concentration buffersolution are used to keep the cell structure of microbes and prevent the cell structure from be ruptured in order to protect the integrity of nucleic acids in cells; and the microbe sample preservation solution contains no high-toxicity components or volatile components, so no volatile poisoning occurs during use.
Owner:知几未来(成都)生物科技有限公司
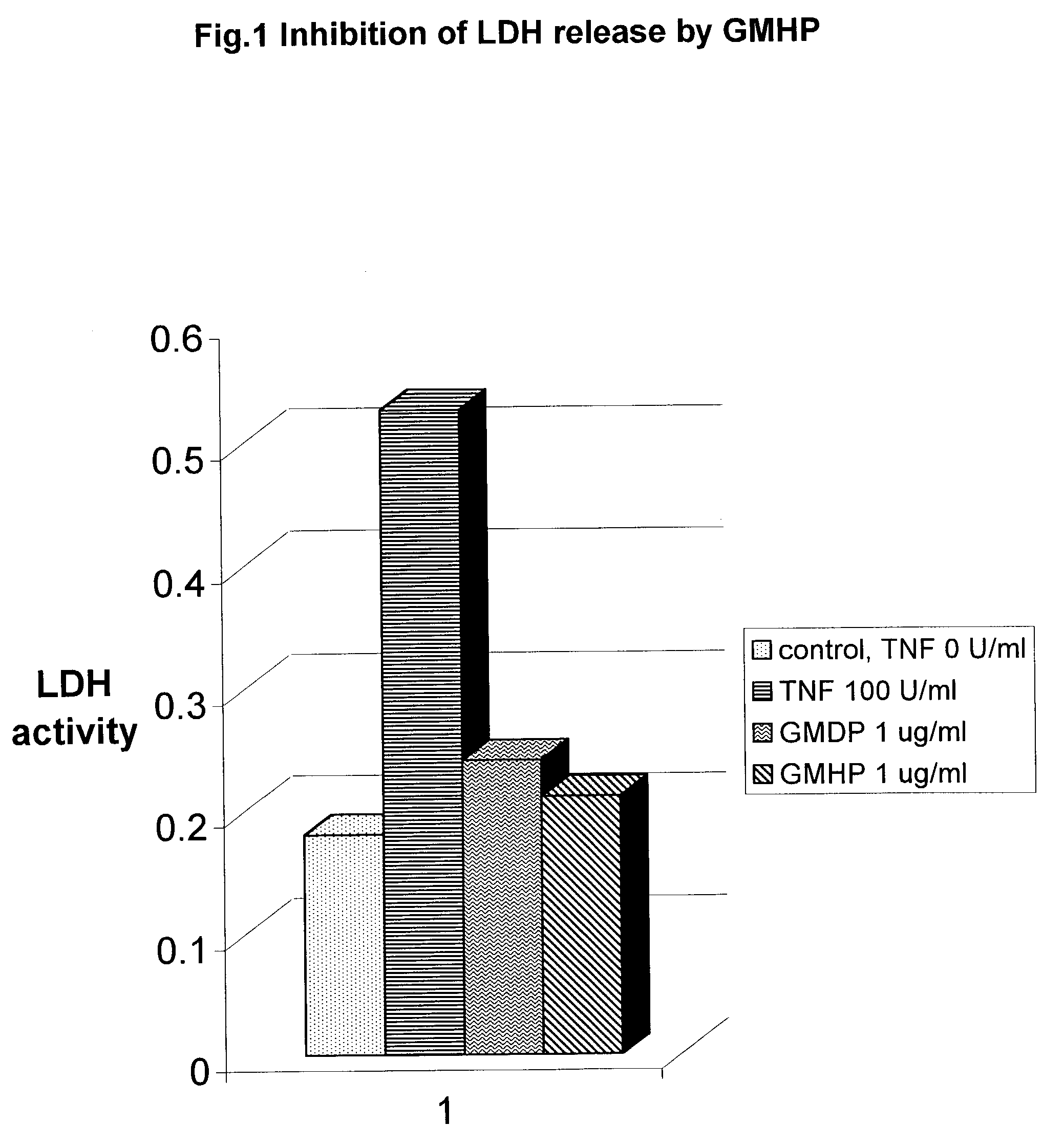
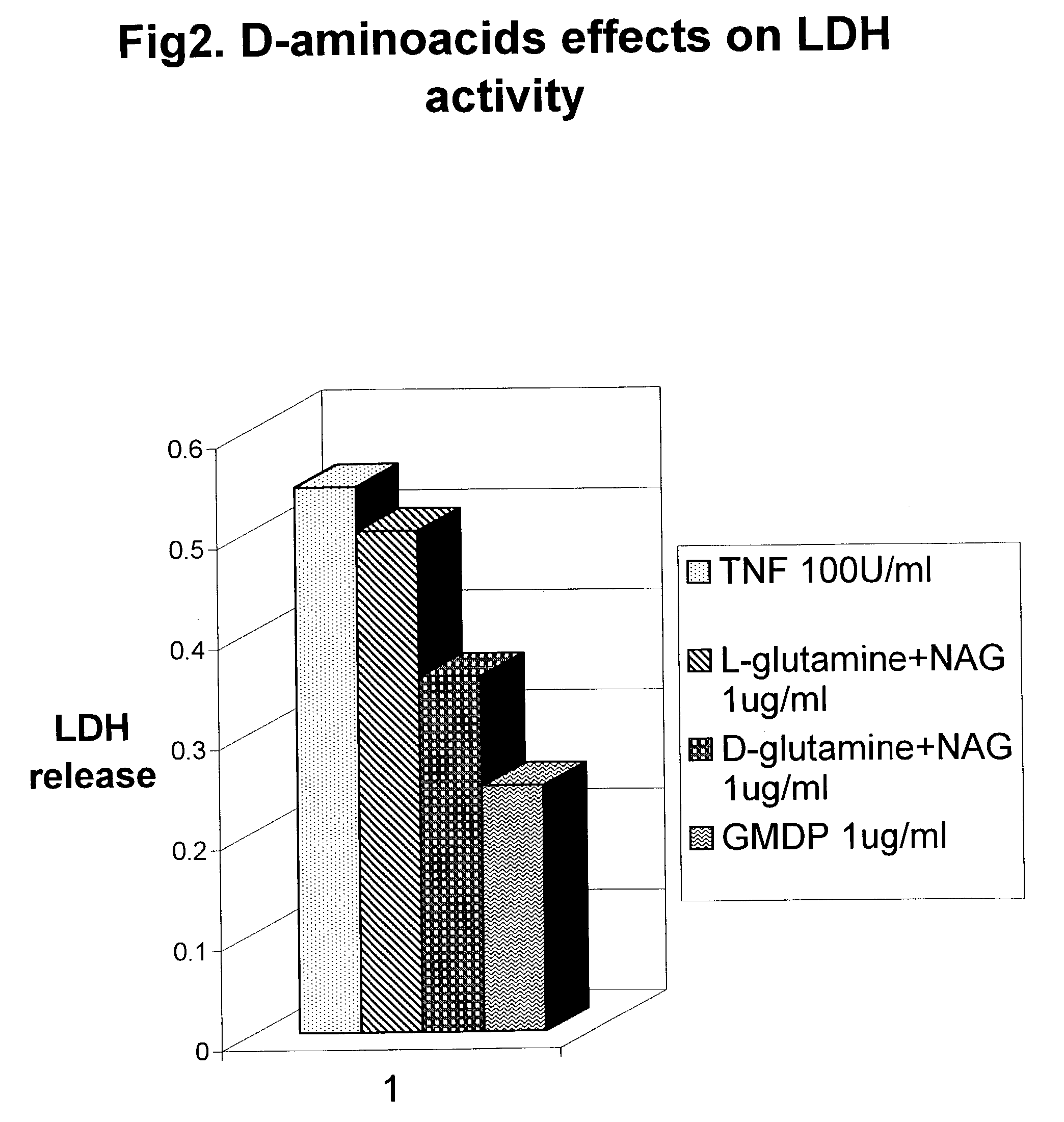
Biodegradable glucosaminemuramyl peptides for apoptosis modulation
InactiveUS7112564B2Low toxicityElevation of LDH and cachexiaPeptidesSaccharide peptide ingredientsCross-linkCytotoxicity
The endopeptidase hydrolysis of cross link peptide bond of the peptidoglycans results in release of the novel glucosaminemuramyl tri, tetra, penta, hexa, and octapeptides. Their structure is defined by specific endopeptidase cleavage as well as genus of gram positive bacteria. They are potent cytoprotective agents capable of inhibiting of TNF alpha cytotoxicity.
Owner:ZYLACTA CORP
Methods for generating the inner ear and other cranial placode-derived tissues using pluripotent stem cells
ActiveUS20150125953A1Epidermal cells/skin cellsNervous system cellsHair cell differentiationSignalling pathways
Disclosed herein are methods and compositions for generating cultures and isolated cell populations containing preplacodal ectoderms cells, otic placode cells, and inner ear sensory hair cells derived from pluripotent cells by modulating TGFβ, BMP, and FGF signaling pathways under defined culture conditions. Also described are methods for obtaining non-otic placodal tissues from pluripotent stem cells. Methods for identifying agents that induce or enhance differentiation and generation of hair cells are also disclosed. Methods for identifying cytoprotective agents for hair cells are also described.
Owner:INDIANA UNIV RES & TECH CORP
Cytoprotective therapeutic agents for the prevention of reperfusion injury following ischemic stroke
The present invention relates generally to the use of γ-glutamyl antioxidants, particularly γ-glutamyl-cysteine, as cytoprotective agents to prevent reperfusion injury (i.e., hemorrhagic transformation) of the blood-brain barrier during reperfusion following an ischemic stroke. The γ-glutamyl antioxidants can be used alone or used in combination with an agent which inhibits the reverse movement of Na / Ca exchange in the blood-brain barrier such as 2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea methanesulphonate (KB-R7943).
Owner:ROSALIND FRANKLIN UNIVERSITY OF MEDICINE AND SCIENCE
Pyrimidine derivative having cell-protecting activity and use thereof
Disclosed is a prophylactic or therapeutic agent for nerve diseases such as ischemic brain diseases and neurodegenerative diseases or the like or a prophylactic or therapeutic agent for diseases for which an antioxidant activity is effective, which can act as a cell-protecting agent, particularly an inhibitor of cerebral cell injury or cerebral cell death. Specifically disclosed is a compound represented by formula (1), or a pharmaceutically acceptable salt, solvate, hydrate or prodrug thereof. The formula (1) is shown in the description.
Owner:ZENYAKU KOGYO KK
Brain cell or nerve cell-protective agents comprising ginsenoside Rb1
InactiveUS20020002141A1Efficient use ofConvenience economicalBiocideNervous disorderDiseaseApoptosis
The present invention provides preparations for efficaciously administering ginsenoside Rb1 or its salt useful as cytoprotective agents. More particularly, the present invention provides pharmaceutical compositions comprising ginsenoside Rb1 or its salt for inhibiting apoptosis or apoptosis-like cell death or pharmaceutical compositions comprising ginsenoside Rb1 or its salt for promoting the expression of a cell death-inhibitory gene product Bcl-xL. Further, the present invention provides preparations for intravenous administration comprising ginsenoside Rb1 or its salt. The above pharmaceutical compositions contain ginsenoside Rb1 or its salt at low extracellular concentrations in lesion, preferably at 1 ng / ml or less and still preferably at 1 to 100 fg / ml. These compositions promote the expression of the cell death-inhibitory gene product Bcl-xL and inhibit apoptosis or apoptosis-like cell death. The above preparations for intravenous administration are useful for therapy, prevention or treatment of many diseases, in particular, brain and nervous diseases.
Owner:JAPAN SCI & TECH CORP
Agent for preventing or improving decline in brain function
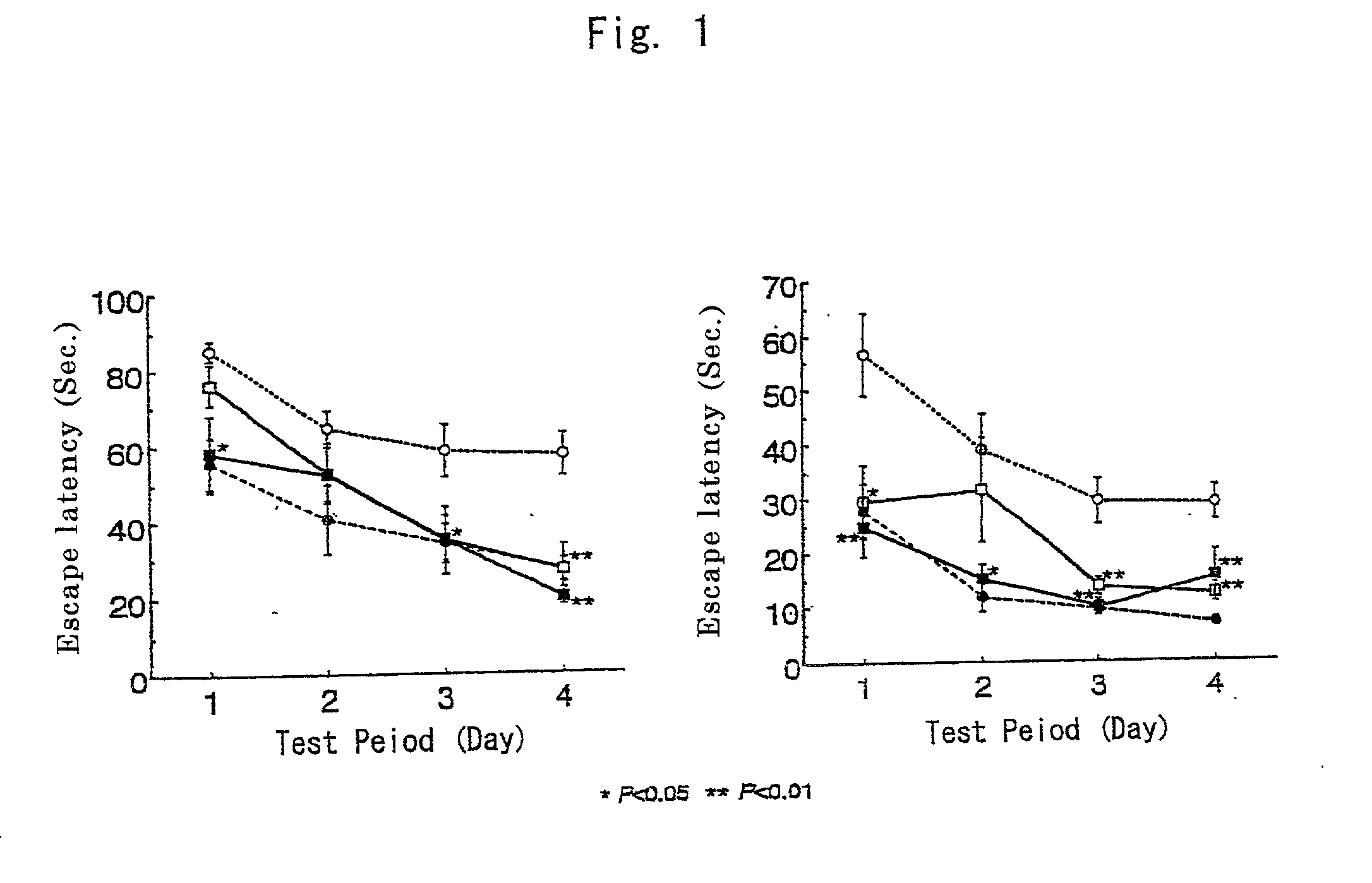
ActiveUS10905705B2Preventing and improving decline in brain functionMemory behavior can be synergistically and effectively improvedOrganic active ingredientsNervous disorderCitrullineExercise performance
An objection of the present invention is to provide an agent for preventing or improving decline in brain function such as decreased perception ability, decreased memory learning ability, decreased thinking ability, decreased concentration, decreased attention, decreased judgment ability, depression, and decreased exercise performance caused thereby. According to the present invention, an agent for protecting brain neuronal cells, comprising citrulline or a salt thereof and citicoline or a salt thereof as active ingredients as well as an agent for preventing or improving decline in brain function, comprising citrulline or a salt thereof and citicoline or a salt thereof as active ingredients is provided.
Owner:KYOWA HAKKO BIO CO LTD
Vagina microbial immunofluorescence staining solution
PendingCN114199653AExtended retention timeImprove permeabilityPreparing sample for investigationBiological testingBiotechnologyImmunofluorescence staining
The invention relates to the technical field of medical detection, and discloses a vagina microorganism immunofluorescence staining solution. Comprising the following raw materials in parts by weight: 5-8 parts of a fluorescent coloring agent, 2-3 parts of an auxiliary dye, 1-3 parts of a stabilizer, 1-3 parts of a penetrant, 2-5 parts of a bacteriostatic agent, 1-2 parts of an anti-quenching agent, 2-4 parts of a permeable agent, 2-4 parts of a buffer solution, 5-6 parts of a cell protective agent and 20-40 parts of purified water. According to the vagina microbial immunofluorescence staining solution, the stability of the fluorescent staining solution during staining can be improved by adding the stabilizer, the fluorescence retention time is prolonged, the problem that the staining fluorescence is not obvious due to poor staining stability and the detection data is influenced is avoided, and meanwhile, the antibacterial activity of the fluorescent staining solution is improved by adding the bacteriostatic agent; the fluorescent staining solution can be stored for a longer time, the permeability agent and the penetrant added in the fluorescent staining solution can improve the permeability to cell membranes, staining of cells by fluorescein can be accelerated, and the practicability of the vagina microbial immunofluorescence staining solution is enhanced.
Owner:济南德亨医学科技有限公司
Fast Acting Inhibitor of Gastric Acid Secretion
The present invention relates to the use of pharmaceutically acceptable zinc salts, in particular, zinc chloride and zinc acetate, alone or optionally, in combinations with one or more of a protein pump inhibitor (PPI), H2 blocker, anti-H. pylori antibiotic / antimicrobial, cytoprotective agent or a combination agent as otherwise described herein for providing fast action with optional long duration effect in reducing gastric acid secretion, including acid secretion in the fundus (by inhibiting vacuolar H+-ATPase or H+ / K+-ATPase) and upper body region of the stomach (by inhibiting H+ / K+-ATPase), thus raising the pH of gastric juices in rapid fashion and decreasing the duration of stomach acid release during a secretagogue phase. This method is also directed to treating conditions including gastroesophageal reflux disease (GERD), non-erosive reflux disease (NERD), Zollinger-Ellison syndrome (ZE disease), ulcer disease, and gastric cancer, as well as preventing or reducing the likelihood of ulcer disease. In addition, the present methods are useful for treating patients who are non-responsive to proton pump inhibitors (PPI) and as an alternative to traditional therapies or conditions which are caused by rapid and complete inhibition of secretagogue induced acid secretion.
Owner:YALE UNIV
Prophylactic or therapeutic agent for hypoxic injury, ischaemia-reperfusion injury and inflammation, cell protection agent for transplantation, and bio-preservation agent
PendingCN111918649AIncrease chances of survivalImproved prognosisAntibacterial agentsNervous disorderReperfusion injuryBULK ACTIVE INGREDIENT
The present invention provides: a prophylactic or therapeutic agent for hypoxic injury, ischaemia-reperfusion injury and inflammation; a cell protection agent for transplantation; and a bio-preservation agent. A prophylactic or therapeutic agent for hypoxic injury, ischaemia-reperfusion injury and inflammation, a cell protection agent for transplantation, and a bio-preservation agent comprising, as an active ingredient, at least one selected from: a heterocyclic compound represented by formula (I) (where the symbols in the formula are as defined in the description) or a salt thereof; an isothiocyanate compound represented by formula (II) S=C=N-R5 (where the symbol in the formula is as defined in the description); and a TRPA1 agonist.
Owner:脑科学香料株式会社
Cytoprotective composition comprising hesperidin or pharmaceutically acceptable salt thereof as an active ingredient
ActiveUS20090227526A1Protect from harmBiocideCarbohydrate active ingredientsBULK ACTIVE INGREDIENTActive ingredient
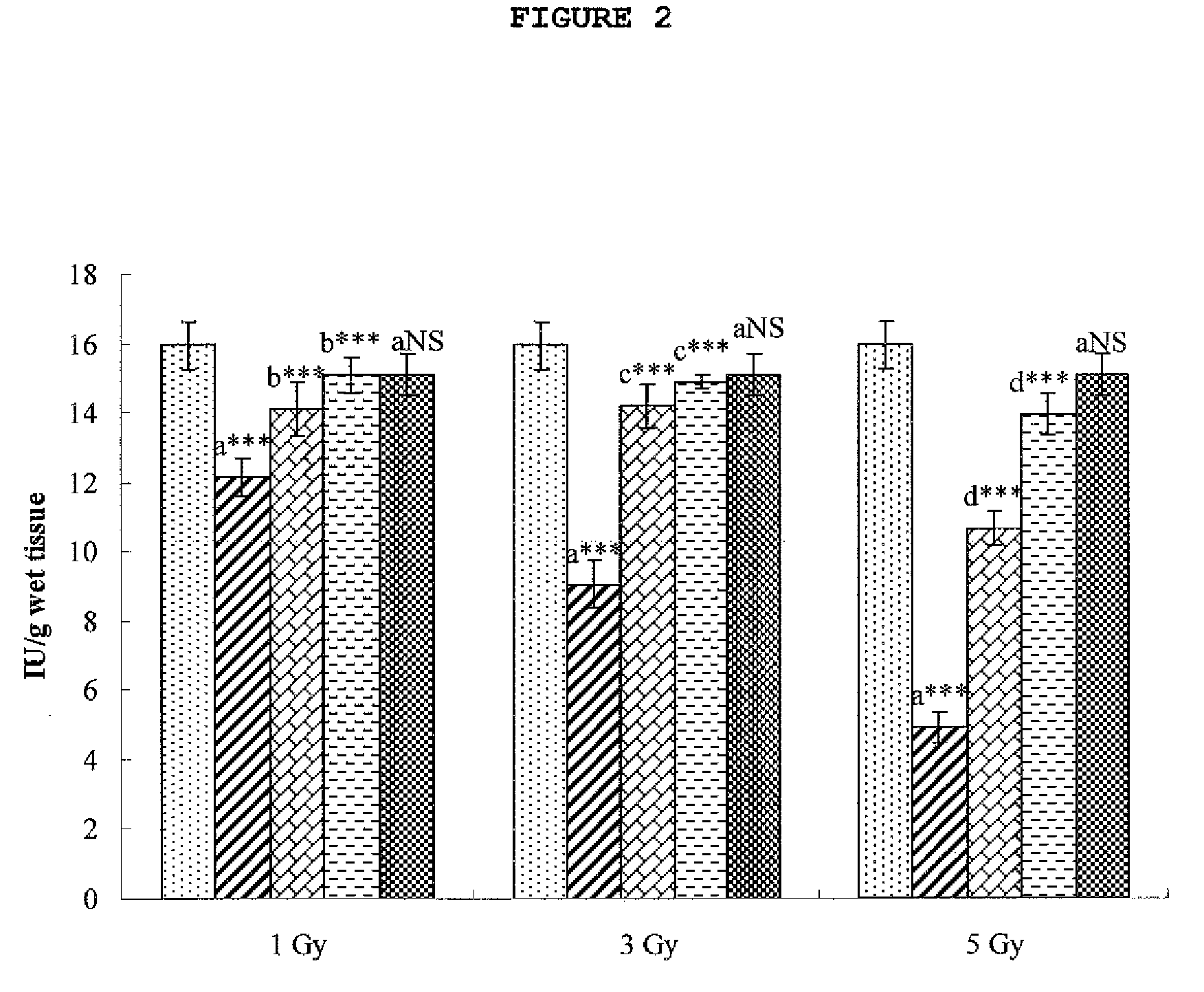
Disclosed is a cytoprotective composition comprising hesperidin or a pharmaceutically acceptable salt thereof as an active ingredient. Having the ability to protect cells from radiation-induced injuries in addition to being non-toxic to cells, hesperidin in accordance with the present invention can be used as an active cytoprotective agent.
Owner:KOREA ATOMIC ENERGY RES INST
Diphenyl substituted cycloalkanes, compositions containing such compounds and methods of use
InactiveCN101137376ASenses disorderNervous disorderAnti asthmatic5-Lipoxygenase-Activating Protein Inhibitors
The instant invention provides compounds of Formula (I) which are 5-lipoxygenase activating protein inhibitors. Compounds of Formula (I) are useful as anti-atherosclerotic, anti-asthmatic, anti-allergic, anti-inflammatory and cytoprotective agents.
Owner:MERCK & CO INC +1
Therapeutic peptides
The present disclosure relates to the field of cytobiology and modulation of cellular mechanisms to control cell viability, cell proliferation and metabolic processes. More specifically, disclosed herein are peptides that are effective in modulating cellular mechanisms that control cell viability, cell proliferation and metabolic processes, including cellular signaling associated with abnormal cell proliferation and malignant diseases. Also disclosed herein are peptides that effectively modulate cellular mechanisms that control cell viability, treat metabolic diseases, and act as cytoprotective agents. Also disclosed herein are peptides useful as apelin receptor agonists.
Owner:COHBAR
Brain cell- or nerve cell-protecting agents comprising medicinal ginseng
InactiveUS20080020988A1Excellent brain cell and nerve cell-protecting effectHigh expressionOrganic active ingredientsBiocideDiseaseMetabolite
The present invention provides medicinal or pharmaceutical compositions and preparations for administration, which are useful as cytoprotective agents and a remedy for neurotrauma, comprising ginseng, its extract, ginseng components, metabolites thereof or salts thereof (for example, red ginseng powder or components thereof). More particularly, the present invention provides medicinal or pharmaceutical compositions for inhibiting apoptosis or apoptosis-like cell death, medicinal or pharmaceutical compositions for promoting the expression of a cell death-suppressing gene product BCl-xL, or preparations for oral or intravenous administration, comprising ginseng, its extracts, ginseng components, metabolites thereof or salts thereof preferably at low concentrations. These medicinal or pharmaceutical compositions and / or preparations for administration are characterized by containing, as the active ingredient(s), ginseng, its extracts, ginseng components, metabolites thereof or salts thereof at low concentrations. These drugs are useful for therapy, prevention or treatment of brain and nervous diseases, heart diseases, etc.
Owner:JAPAN SCI & TECH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![[1,2,3] triazolyl substituted quinolines and coumarins as inhibitors of leukotriene biosynthesis [1,2,3] triazolyl substituted quinolines and coumarins as inhibitors of leukotriene biosynthesis](https://images-eureka.patsnap.com/patent_img/e807bd41-5a7a-43a5-8d6d-71607587208f/A2008800112240119H1.PNG)
![[1,2,3] triazolyl substituted quinolines and coumarins as inhibitors of leukotriene biosynthesis [1,2,3] triazolyl substituted quinolines and coumarins as inhibitors of leukotriene biosynthesis](https://images-eureka.patsnap.com/patent_img/e807bd41-5a7a-43a5-8d6d-71607587208f/A2008800112240120H1.PNG)
![[1,2,3] triazolyl substituted quinolines and coumarins as inhibitors of leukotriene biosynthesis [1,2,3] triazolyl substituted quinolines and coumarins as inhibitors of leukotriene biosynthesis](https://images-eureka.patsnap.com/patent_img/e807bd41-5a7a-43a5-8d6d-71607587208f/A2008800112240121H1.PNG)