Therapeutic peptides
A non-polar and polar side chain technology, applied in the field of cell biology, can solve the problems of natural occurrence and function is not determined, the potential biological activity of exogenous peptide is unknown, structure prediction and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0310] The embodiments listed below are presented in numbered form for ease and clarity of reference when referring back to the various embodiments.
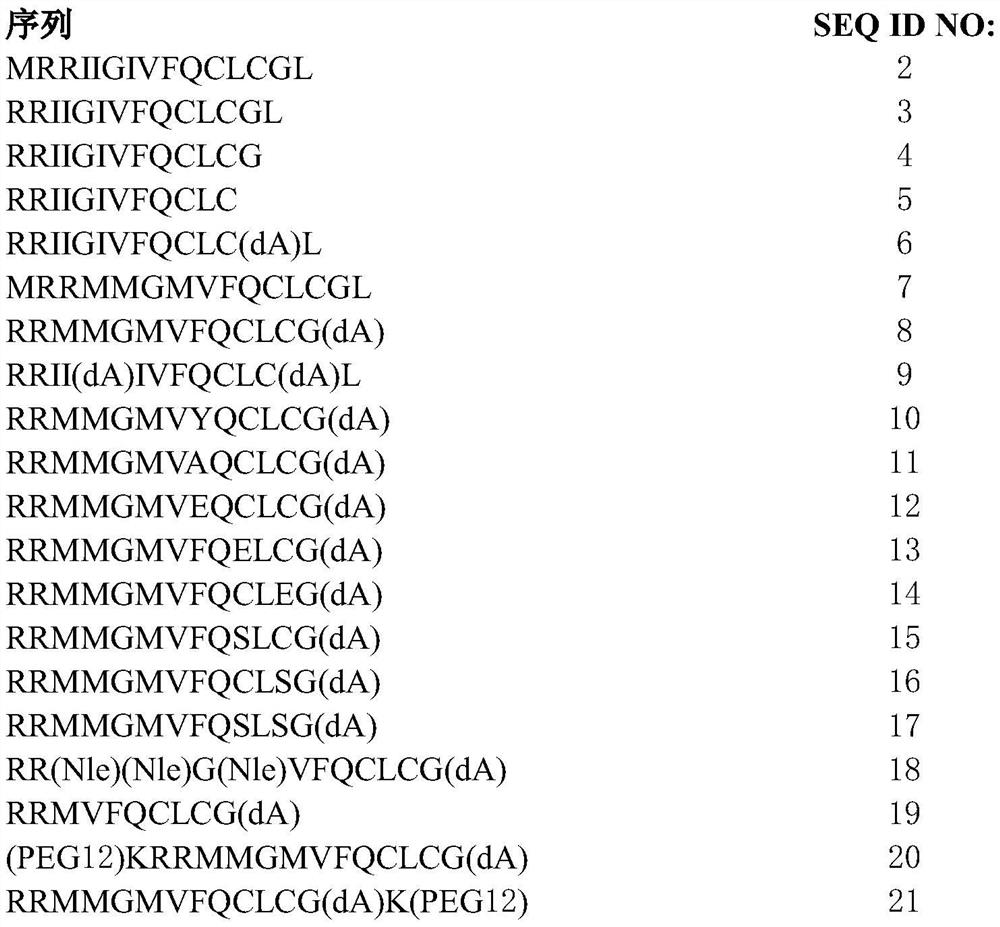
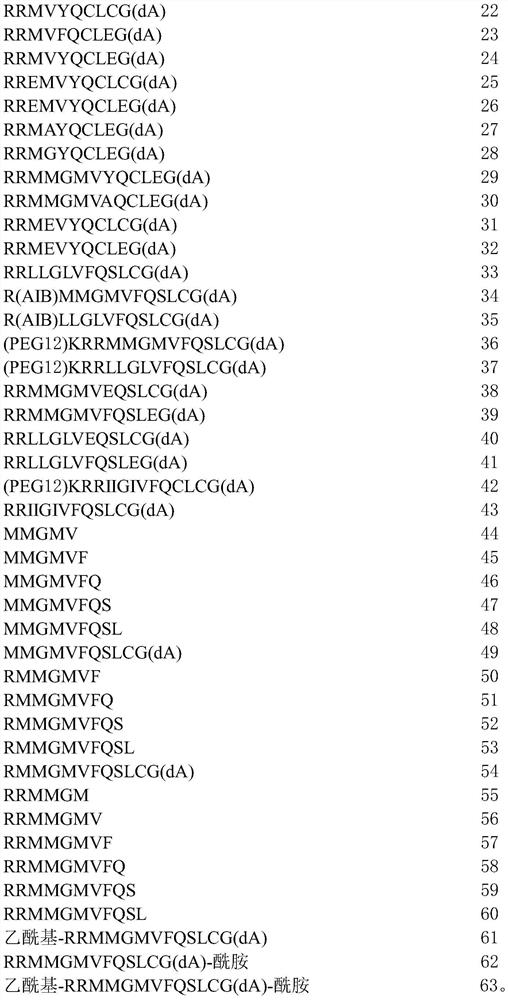
[0311] 1. A peptide comprising the amino acid sequence of formula I:
[0312] x 1 -RX 2 -X 3 -X 4 -X 5 -X 6 -Q-X 7 -L-X 8 -X 9 (I) (SEQ ID NO: 1)
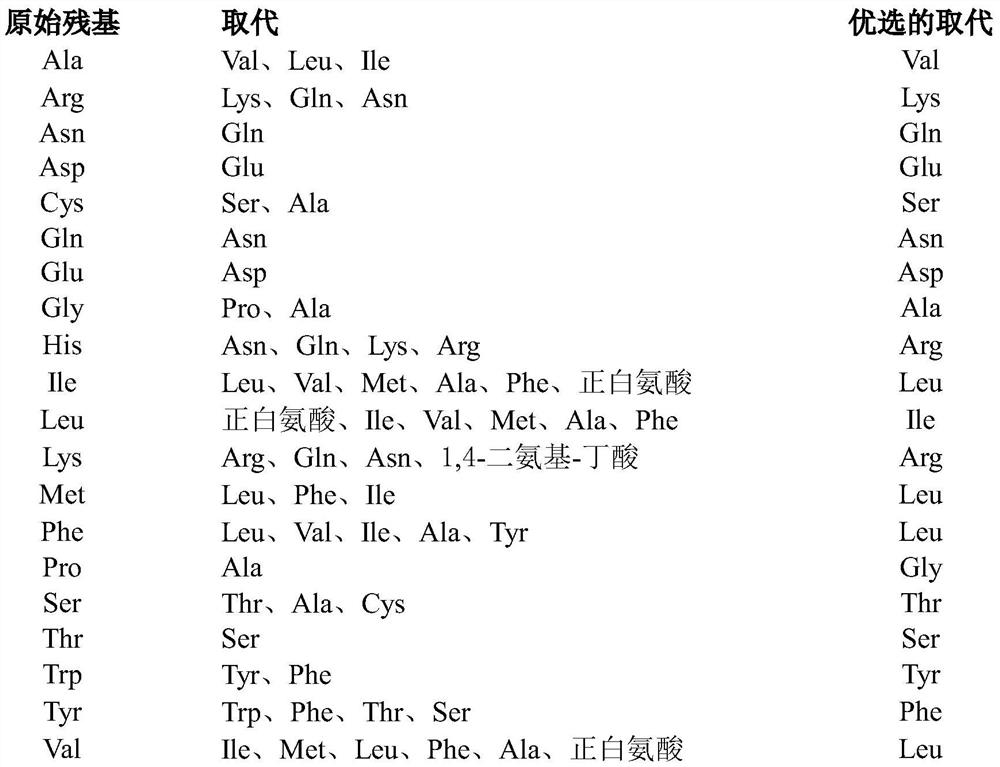
[0313] where X 1absent, or if present, an amino acid with a polar side chain or a non-polar side chain; X 2 is an amino acid with a polar side chain or a non-polar side chain; X 3 is absent, or if present, then one to three amino acids, each amino acid independently having a polar side chain or a non-polar side chain; X 4 is an amino acid with a polar side chain or a non-polar side chain; X 5 is an amino acid with a non-polar side chain; X 6 is an amino acid with a polar side chain or a non-polar side chain; X 7 is an amino acid with a polar side chain; X 8 is an amino acid with a polar side chain; and X 9 Absent, or if present, from one to three amino acids, each a...
example
[0381] Example 1 - Synthesis
[0382]Unless otherwise specifically stated, peptides were prepared via solid-phase synthesis using t-Boc or Fmoc chemistry or other well-established techniques on suitable resins by methods analogous to those described below (see, e.g., Stewart and Young, Solid Phase Peptide Synthesis (Solid Phase Peptide Synthesis), Pierce Chemical Co., Rockford, IL (Rockford, III.), 1984; E. Atherton and R.C. Sheppard, "Solid Phase Peptide Synthesis. A Practical Approach )", Oxford-IRL Press, New York (New York), 1989; Greene and Wuts, "Protective Groups in Organic Synthesis (Protective Groups in Organic Synthesis)", John Wiley & Sons, 1999; Florencio Zaragoza Dorwald, "Solid Phase Organic Synthesis ( Organic Synthesis on solid Phase), Wiley-VCH Verlag GmbH, 2000; and "Fmoc Solid Phase Peptide Synthesis", edited by W.C. Chan and P.D. White, Oxford University Press, 2000).
[0383] Solid phase synthesis is initiated by attaching the N-terminally protected amino...
example 2
[0390] Example 2 - Caspase 3 / 7 Activity
[0391] The effect of peptides on cell death / survival can be assessed using a caspase-3 assay. Peptides were dissolved in DMSO to obtain 10 mM stock solutions. Staurosporine was used as a highly effective positive control for caspase induction. Staurosporine (Selleckchem) was dissolved in DMSO to obtain a 1 mM stock solution. Caspase-Glo 3 / 7 assay reagents were purchased from Promega (Madison, WI). The MDA-MB-231 human breast cancer cell line was purchased from the American Type Culture Collection (Manassas, VA). MDA-MB-231 cells were grown in DMEM medium supplemented with 10% FBS. 100 μg / ml penicillin and 100 μg / ml streptomycin were added to the medium. Cultures were maintained at 37 °C with 5% CO 2 and 95% air in a humid atmosphere. MDA-MB-231 cells were incubated in duplicate at 37°C for 18 hours with 10 μΜ of the test peptide in a humidified atmosphere of 5% CO2 and 95% air. 25 μl of Caspase-Glo 3 / 7 Assay Reagent was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com