LOOP PEPTIDE AND TGF alpha FOR STIMULATING STEM CELL PROLIFERATION AND MIGRATION
a technology of stem cell proliferation and migration, which is applied in the direction of drug compositions, immunological disorders, metabolism disorders, etc., can solve the problems of life-threatening hemorrhage in patients already immuno-compromised, breakdown of gi barrier function and septic condition, and reduce the tolerated dose, so as to stimulate hematopoiesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Loop Peptide
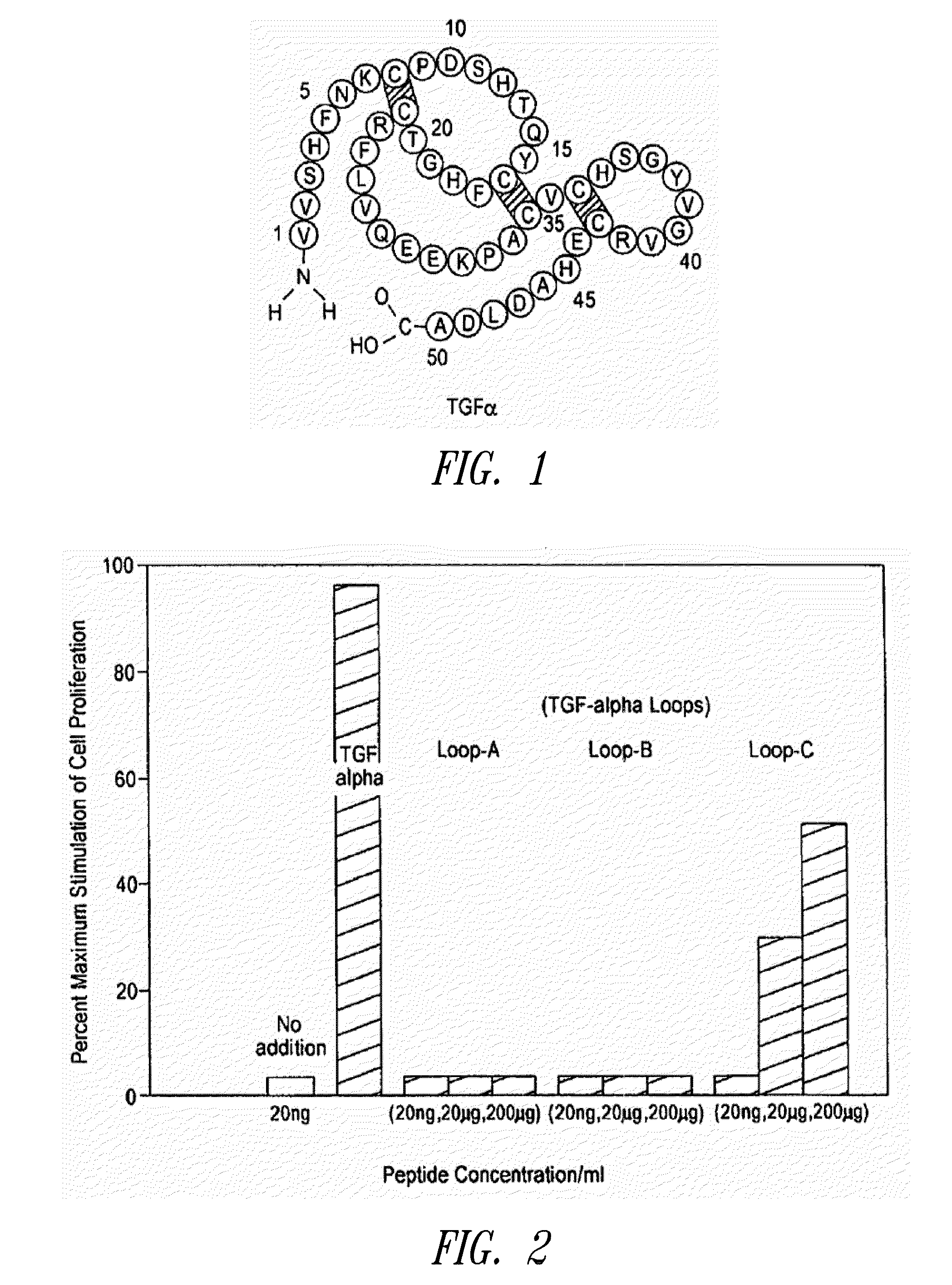
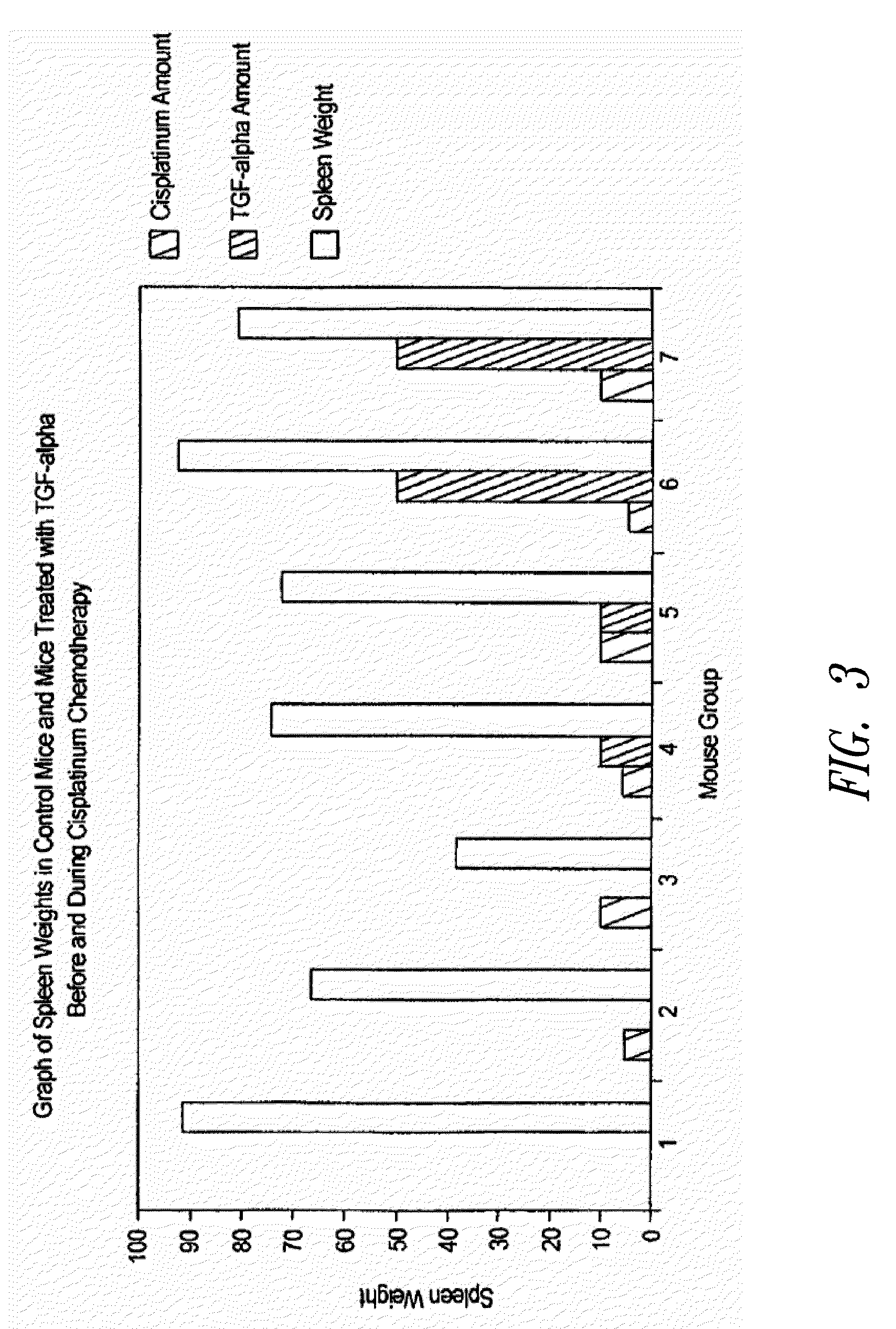
[0032]Human TGFα is a polypeptide of 50 amino acids and the corresponding rat sequence is shown in FIG. 1. The human or rat TGFα polypeptide can be divided roughly into three loop regions corresponding roughly (starting at the N terminus) to amino acids 1-21, to amino acids 16-32, and to amino acids 33-50. Each of the three foregoing loop regions in human TGFα was investigated for TGFα-like biological activity, such as stimulation of cellular proliferation as measured by 3H thyridine incorporation of stem cells. As shown in FIG. 2, only the Loop C peptide (corresponding to amino acids 33-50) showed significant TGFα biological activity and is therefore a TGFα mimetic peptide. Therefore, in view of the fact that the loop peptide exhibited TGFα biological activity, data obtained with TGFα (50 amino acid polypeptide or even the altered splice 57 amino acid polypeptide) is predictive. Accordingly, data from TGFα or TGFα 57 show what can be called “TGFα activity” and these are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com