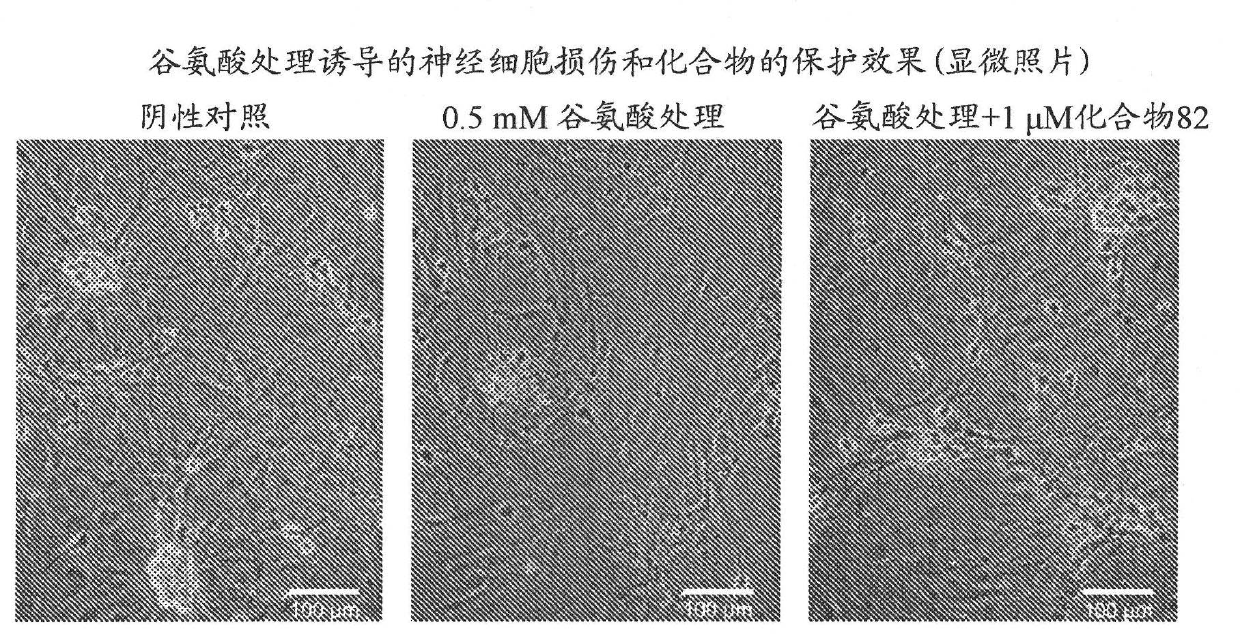
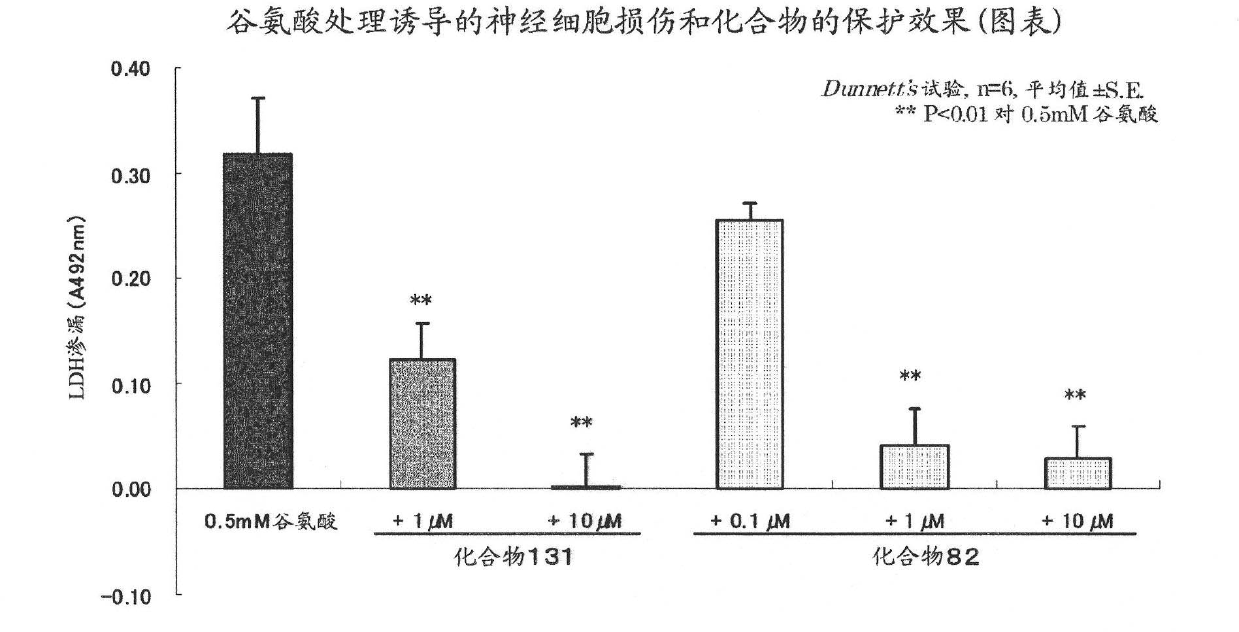
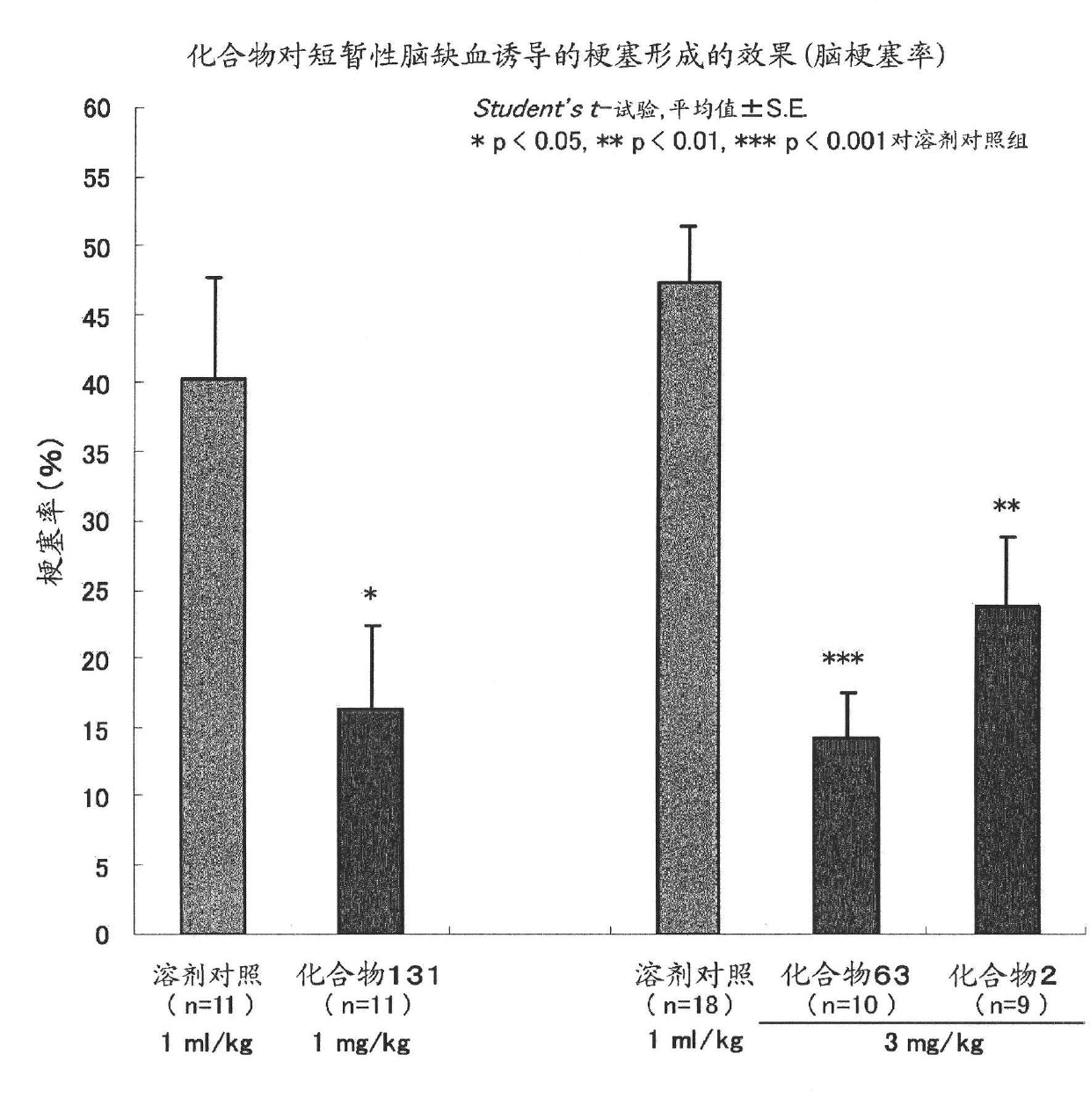
Pyrimidine derivative having cell-protecting activity and use thereof
A technology for drugs and compounds, applied in the field of novel pyrimidine derivatives, can solve problems such as insufficient effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0125] One mode of embodiment of the present invention is a compound represented by general formula (1a), or a pharmaceutically acceptable salt, solvate, hydrate or prodrug thereof:
[0126] [chemical formula 11]
[0127]
[0128] where R 1 , R 2 and R 3 Each independently selected from general formula (2a), or one of them is replaced by 1 or 2 R 5 Substituted amino, or phenyl (C1-C6) alkyl, the other two are independently selected from the general formula (2a),
[0129] and R 4 Represents -F, -Cl, -Br, -I, formyl, phenyl or (C1-C6) alkoxy, wherein the phenyl can be replaced by 1 or 2 R 6 replace,
[0130] [chemical formula 12]
[0131]
[0132] wherein m is 0 or 1; n is 1, 2 or 3;
[0133] R 5 Represents -H, carboxy, (C1-C6) alkyl, (C1-C6) alkoxycarbonyl, (C1-C6) alkoxycarbonylmethyl, amino (C1-C6) alkyl (the amino group may be 1 or 2 (C1-C6) alkyl, or 1 (C1-C6) alkoxycarbonyl substituted; in addition, the carbon chain may contain carboxyl), piperazinyl (C1-C6...
Embodiment approach 2
[0303] A different embodiment of the present invention is a cytoprotective agent comprising a compound represented by general formula (1b), or a pharmaceutically acceptable salt, solvate, hydrate or prodrug thereof:
[0304] [chemical formula 18]
[0305]
[0306] where R 1 , R 2 and R 3 Each independently selected from general formula (2b):
[0307] [chemical formula 19]
[0308]
[0309] wherein m is 0 or 1; n is 1, 2 or 3;
[0310] R 5 Represents -H, (C1-C6) alkyl, (C2-C6) alkenyl, (C2-C6) alkynyl or phenyl, wherein the phenyl can be replaced by 1 or 2 R 6 further replace;
[0311] R 6 Represents -H, -F, -Cl, -Br, -I, (C1-C6) alkyl, (C1-C6) alkylamino, (C1-C6) alkoxy, (C1-C6) alkylthio , (C1-C6) acyl, pyrrolidinyl, piperidinyl, piperazinyl, (C1-C6) alkoxycarbonyl, phenyl, benzyl, phenyl (C1-C6) alkoxy, nitro, Amino or hydroxyl;
[0312] Ar means can be replaced by 1 or 2 R 6 Substituted phenyl, benzyl, pyridyl, pyrimidinyl, thienyl, pyrrolyl, quinolinyl, t...
Embodiment 1
[0486] 4,6-Dimorpholino-2-(4-phenylpiperazin-1-yl)pyrimidine (compound 1)
[0487] (1-1) 4,6-dichloro-2-(4-phenylpiperazin-1-yl)pyrimidine and 2,4-dichloro-6-(4-phenylpiperazin-1-yl)pyrimidine Synthesis
[0488] (See the following reaction formula (11))
[0489] [chemical formula 25]
[0490]
[0491] The synthesis method will be specifically described in the following order. 2,4,6-Trichloropyrimidine (20ml, 165mmol) and triethylamine (27.7ml) were dissolved in ether (200ml), and phenylpiperazine (25.2ml, 165mmol) was added under ice cooling. After stirring at room temperature for about 1 hour, water was added and extracted twice with ether. The ether layer was washed with saturated brine, dried over magnesium sulfate, and the solvent was distilled off under reduced pressure. Purification was performed by silica gel chromatography (hexane:ethyl acetate=19:1) to obtain 17.6 g of 4,6-dichloro-2-(4-phenylpiperazin-1-yl)pyrimidine (yield 34%). The NMR data of the obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com