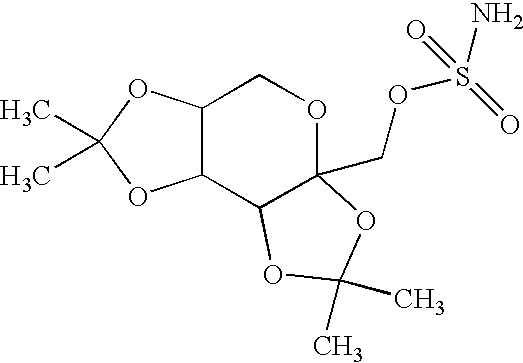
Sustained release topiramate
a technology of topiramate and sucralose, which is applied in the field of sucralose, can solve the problems of manic depression, higher incidence of bipolar affective disorder, and inability to maintain the effect of preventing the degradation or growth of contaminants, and facilitating manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0093] This example illustrates a method of administering a mania-alleviating amount of topiramate in a sustained-release formulation.
[0094] A dose of topiramate (e.g., 400 mg) in a sustained-release formulation is administered to the patient for at least four to six weeks. The Patient is monitored for a decrease in symptoms of mania as measured by the AMDS Mania-Depression Scale (Woggen, B., Int. Pharmacopsychiat., 14:228-242, 1979; Woggen, B., Int. Pharmacopsychiat., 14:325-337, 1979) or the Manic State Rating Scale (MSRS; Beigel, A., et al., Arch. Gen. Psychiat. 25:256-262, 1971; Cookson, J. C., et al., Neuropharmacology 18:1011-1013, 1979). The AMDS and MSRS will show clinical improvement in the symptoms of mania.
example 2
[0095] This example illustrates a method of administering a mania-alleviating amount of topiramate in a sustained-release formulation.
[0096] This experiment utilizes two test groups of patients. All of the patients are suffering from mania. The study is a randomized, double-blind, placebo controlled design. One treatment group receives a dose (e.g., 400 mg) of sustained-release topiramate, the other group receives a placebo. A dose of placebo or topiramate (e.g., 400 mg) in a sustained-release formulation is administered once a day to the patients for at least 4 weeks by the patients or medical professional. Efficacy is measured by the AMDS Mania-Depression Scale (Woggen, B., Int. Pharmacopsychiat., 14:228-242, 1979; Woggen, B., Int. Pharmacopsychiat., 14:325-337, 1979) or the Manic State Rating Scale (MSRS; Beigel, A., et al, Arch. Gen. Psychiat. 25:256-262, 1971; Cookson, J. C., et al., Neuropharmacology 18:1011-1013, 1979). Results are compared between the test group and the plac...
example 3
[0097] This example illustrates the bio-equivalency with respect to the extent of absorption of the sustained-release topiramate as compared to administration of topiramate conventional immediate release tablet when administered as multiple daily doses.
[0098] In this study two groups of twenty human subjects are given a dose of 800 mg of topiramate either as 1) a single sustained-release dose, as embodied by the present invention, 2) conventional immediate release tablets of 200 mg administered four times a day or 400 mg tablets administered twice a day. Plasma concentrations of topiramate are measured serially to define the topiramate plasma concentration-time profile by published methods including gas chromatography (Riffitts, et al., A capillary gas chromatographic assay with nitrogen phosphorus detection for the quantification of topiramate in human plasma, urine and whole blood, J. Pharm. Biomed. Anal. 19:363-371, 1999) and HPLC (Gidal and Lensmeyrer, Therapeutic monitoring of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com