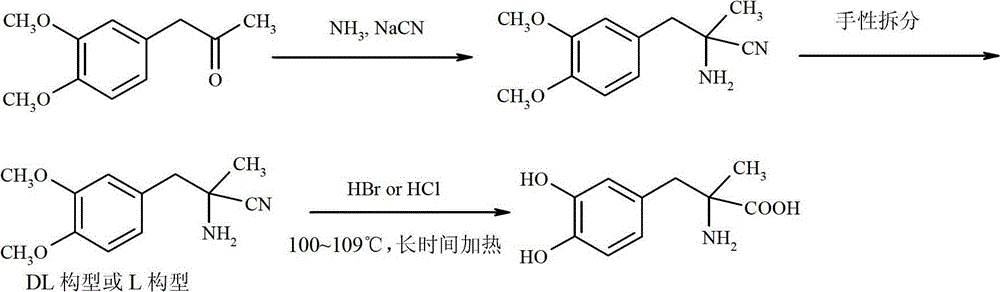
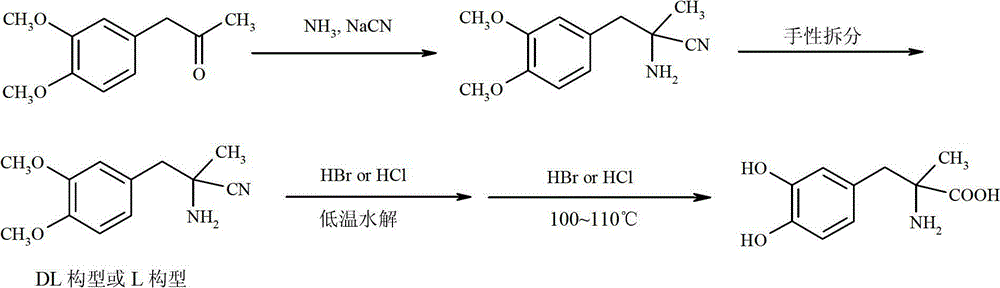
Preparation of Methyldopa by Hydrolysis of α-Methyl-(3,4-Dimethoxyphenyl)-α-aminopropionitrile by Two-step Hydrolysis
A technology of dimethoxyphenyl and methyldopa, which is used in the preparation of methylpolycarbonate by hydrolyzing α-methyl-(3,4-dimethoxyphenyl)-α-aminopropionitrile by a two-step hydrolysis method In the field of Pakistan, it can solve the problems of high price, increased by-products, and high energy costs, and achieve the effects of reducing energy and economic costs, improving purity and yield, and reducing acid wastewater discharge.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In a three-neck flask equipped with a thermometer, a stirrer and a reflux condenser, add 12.3 grams (0.12 mol) of 36% concentrated hydrochloric acid. Cool to 0~5°C with ice water. Slowly add 2.18 grams (0.0085mol) of DL-2-amino-3-(3,4-dimethoxyphenyl)-2-methyl-propionitrile hydrochloride [or L-2-amino-3-( 3,4-Dimethoxyphenyl)-2-methyl-propionitrile hydrochloride, the same for the following examples] was dissolved in ice-cold concentrated hydrochloric acid. Stir vigorously for 2 hours after the addition is complete. After the reaction was completed, the temperature was raised to room temperature, and N was directly introduced into the reaction mixture slowly. 2 , while extracting excess HCl by distillation under reduced pressure. The solid was washed with 3 x 25ml of acetone to obtain a white solid. It was then vacuum dried at 50°C for 3 hours. It was further dried at 70°C for 5 hours. Obtain product DL-2-amino-3-(3,4-dimethoxyphenyl)-2-methyl-propion hydrochloride...
Embodiment 2
[0041] In a three-neck flask equipped with a thermometer, a stirrer and a reflux condenser, add 24.3 grams (0.24 mol) of 36% concentrated hydrochloric acid. Cool with ice to 0~4°C. Slowly add 14.38 grams (0.056mol) of DL--2-amino-3-(3,4-dimethoxyphenyl)-2-methyl-propionitrile hydrochloride dissolved in ice-cold concentrated hydrochloric acid, and add Afterwards, keep stirring vigorously for 2 hours at 0-4°C. After the reaction was completed, the temperature was raised to room temperature, and N was directly introduced into the reaction mixture slowly. 2 , while extracting excess HCl by distillation under reduced pressure. The solid was washed with 4×25ml of acetone to obtain a white solid. It was then vacuum dried at 50°C for 3 hours. It was further dried at 70°C for 5 hours. 14.70 g of the product DL-2-amino-3-(3,4-dimethoxyphenyl)-2-methyl-propion hydrochloride was obtained, with a yield of 95.2%.
Embodiment 3
[0043] In a three-neck flask equipped with a thermometer, a stirrer and a reflux condenser, add 24.3 grams (0.12 mol) of 18% concentrated hydrochloric acid. Cool to 0~5°C with ice water. Slowly added 2.18 g (0.0085 mol) of DL-2-amino-3-(3,4-dimethoxyphenyl)-2-methyl-propionitrile hydrochloride dissolved in ice-cold concentrated hydrochloric acid. Stir vigorously for 2 hours after the addition is complete. After the reaction was completed, the temperature was raised to room temperature, and N was directly introduced into the reaction mixture slowly. 2 , while extracting excess HCl by distillation under reduced pressure. The solid was washed with 3 x 25ml of acetone to obtain a white solid. It was then vacuum dried at 50°C for 3 hours. It was further dried at 70°C for 5 hours. 1.85 g of the product DL-2-amino-3-(3,4-dimethoxyphenyl)-2-methyl-propion hydrochloride was obtained, with a yield of 79.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com