Use of aminopropionitrile in preparation of medicine
An aminopropionitrile, a technology for preparing drugs, applied in the field of biomedicine, can solve the problems of lack of treatment plans for patients with liver fibrosis and cirrhosis, difficulty in predicting the disease process of patients, unapproved and other problems, and achieves improvement in liver cirrhosis, easy absorption, and good quality. water soluble effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
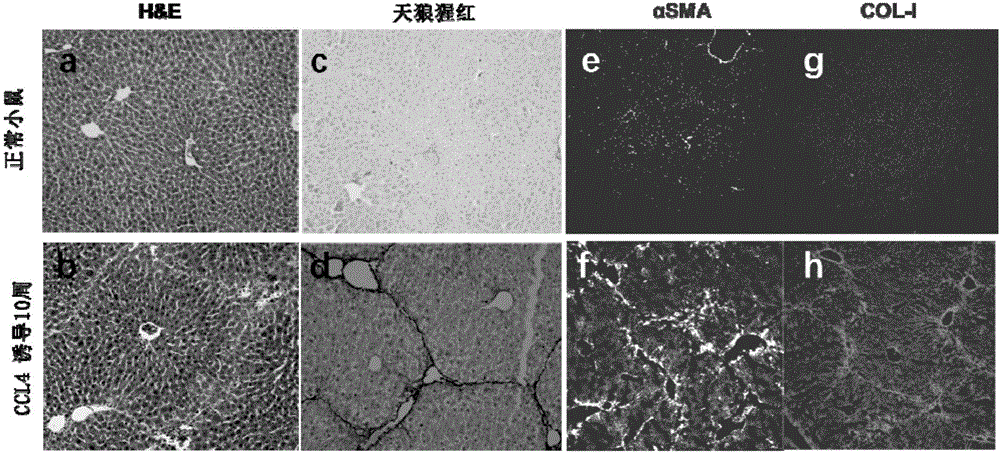
[0041] In this embodiment, 10 healthy experimental mice were induced with chronic liver injury for 10 weeks to obtain experimental mice with severe fibrosis in the liver. Specifically, in this embodiment, each intraperitoneal injection of 2.5mL / kg of CCl was performed according to the weight of the mouse 4 The mixed solution with the volume ratio of 1:4 to olive oil was injected twice a week, and the induction period was 10 weeks. In addition, healthy mice were selected as controls for model construction.
[0042] The results of the histological identification of the liver tissue of the mouse that induced chronic liver injury for 10 weeks in this embodiment, such as figure 1 .b and figure 1 shown in d. The structure and collagen fibers of healthy mouse liver such as figure 1 a and figure 1 as shown in c. figure 1 In .b, the H&E stained liver tissue structure shows obvious portal vein-portal vein bridging, and also portal vein-central vein bridging, indicating that the de...
Embodiment 2
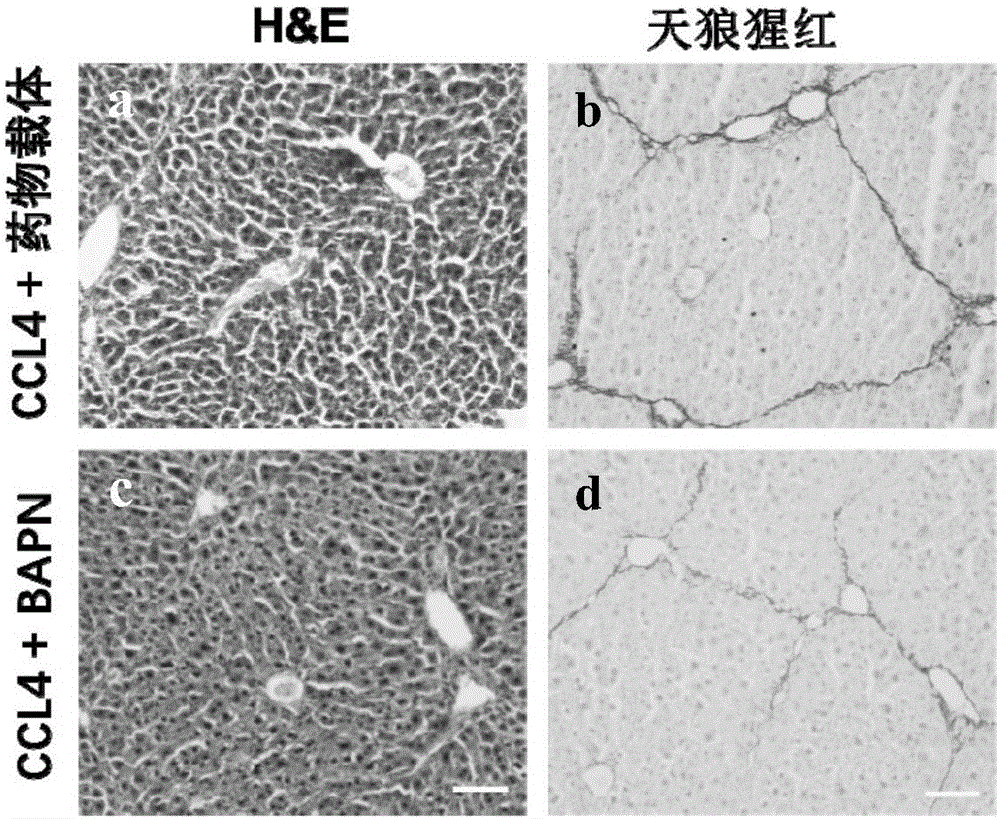
[0046] In this example, the administration experiment of BAPN was carried out on 5 mice with chronic liver injury in Example 1. Specifically, in this embodiment, 100 mg / kg of BAPN was injected intraperitoneally according to the weight of the mice, once a day, and the experimental period was 2 weeks. After 2 weeks, the mice were anesthetized with trichloroethanol, and the liver tissues were collected for analysis.
Embodiment 3
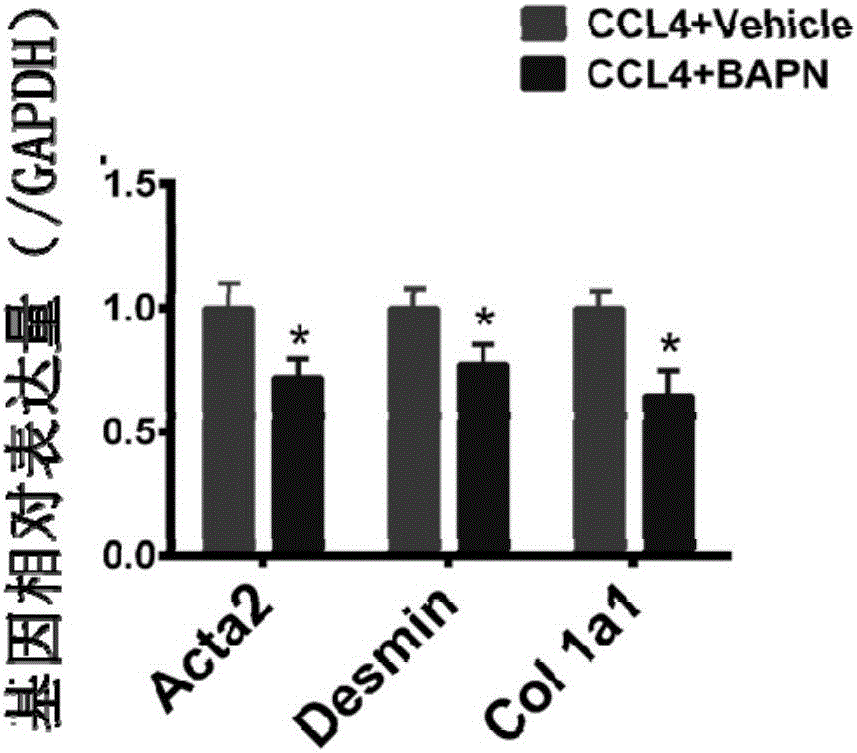
[0050] In this example, for all mouse liver tissues of the BAPN group in Example 2 and all mouse liver tissues of the control group in Comparative Example 1, RNA was extracted and reverse-transcribed into cDNA, and qPCR was performed to detect the expression of fibrosis marker genes. Express the situation. Specifically, in this embodiment, the fibrosis marker genes are selected from col 1a1, Acta2 and Desmin, respectively.
[0051] The expression situation of the fibrosis marker gene of 2 groups of mouse livers of this embodiment, such as figure 2 shown. Depend on figure 2 It can be seen that, compared with the drug carrier (Vehicle)-PBS group of the comparative example, the expressions of the fibrosis marker genes col1a1, Acta2 and Desmin in the BAPN group of Example 2 all decreased. t test (Student's t test) was performed on the difference genes among them, and it was found that there were significant differences in the fibrosis marker genes (*p<0.05, significant differ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com