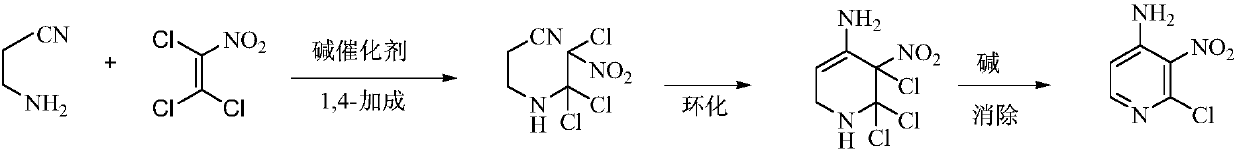
Environmental protection preparation method of 4-amino-2-chloro-3-nitropyridine
A technology of nitropyridine and trichloronitroethylene, applied in the direction of organic chemistry and the like, can solve the problems of inflammability and explosion of recrystallization solvent, limitation of industrial production, large amount of waste acid and waste water, etc., and achieves suitable reaction activity and low cost. , the effect of good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of 4-amino-2-chloro-3-nitropyridine
[0029] In the reactor with stirring, thermometer and reflux system, add 60 kg of tetrahydrofuran, 7.5 kg (107 moles) of 3-aminopropionitrile, 17.5 kg (100 moles) of trichloronitroethylene, 0.1 kg of DBU, 50- Stir and react at 55°C for 4 hours, then cool to 30-40°C, add 30.5 kg (220 moles) of potassium carbonate, stir and react at 55-60°C for 3 hours, then cool to 20-25°C, filter, filter cake with 20 kg of tetrahydrofuran Wash, combine the filtrates, distill and recover the solvent, add 45 kg of isopropanol and 0.5 kg of activated carbon to the residue, decolorize at 55-60 ° C for 1 hour, filter while hot, recrystallize, filter, and dry to obtain 16.2 kg of light yellow solid 4 -Amino-2-chloro-3-nitropyridine, yield 93.4%, gas phase purity 99.8%.
[0030] The NMR data of the product are as follows:
[0031] 1 HNMR (CDCl3, δ, ppm): 8.25 (d, 1H), 7.29 (d, 1H), 4.71 (b, 2H).
Embodiment 2
[0032] Embodiment 2: Preparation of 4-amino-2-chloro-3-nitropyridine
[0033] In the reactor connected with stirring, thermometer and reflux system, add 70 kg of methanol, 7.5 kg (107 moles) of 3-aminopropionitrile, 17.5 kg (100 moles) of trichloronitroethylene, 0.1 kg of 4-dimethylamino Piperidine, stirred and reacted at 35-40°C for 5 hours, then added 10 kg (250 moles) of sodium hydroxide, stirred and reacted at 30-35°C for 2 hours, filtered, the filter cake was washed with 20 kg of methanol, the filtrates were combined, and the solvent was recovered by distillation. Add 45 kg of isopropanol and 0.5 kg of activated carbon to the residue, decolorize at 55-60 ° C for 1 hour, filter while hot, recrystallize, filter, and dry to obtain 15.7 kg of light yellow solid 4-amino-2-chloro-3- Nitropyridine, yield 90.5%, gas phase purity 99.7%.
Embodiment 3
[0034] Embodiment 3: Preparation of 4-amino-2-chloro-3-nitropyridine
[0035]In the reactor connected with stirring, thermometer and reflux condenser, add 60 grams of acetonitrile, 7.5 grams (0.107 moles) of 3-aminopropionitrile, 17.5 grams (0.1 moles) of trichloronitroethylene, 0.1 grams of DBU, 55- Stir and react at 60°C for 4 hours, then cool to 30-40°C, add 12.7 grams (0.12 moles) of sodium carbonate, stir and react at 40-45°C for 4 hours, then cool to 20-25°C, filter, filter cake with 20 grams of acetonitrile Wash, combine the filtrates, distill and recover the solvent, add 45 g of isopropanol and 0.5 g of activated carbon to the residue, decolorize at 55-60 ° C for 1 hour, filter while hot, recrystallize, filter, and dry to obtain 16.0 g of light yellow solid 4 -Amino-2-chloro-3-nitropyridine, yield 92.2%, gas phase purity 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com