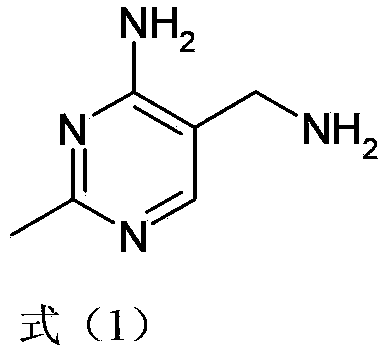
Simple and convenient preparation method of key intermediate (2-methyl-4-amino-5-amino methyl pyrimidine) for vitamin B1
A technology of formylaminomethylpyrimidine and aminomethylpyrimidine, which is applied in the field of production of vitamin B1 and its derivatives, can solve the problems of high reaction temperature, low yield, difficulty in removing o-chloroaniline, etc., and achieve few steps and easy operation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
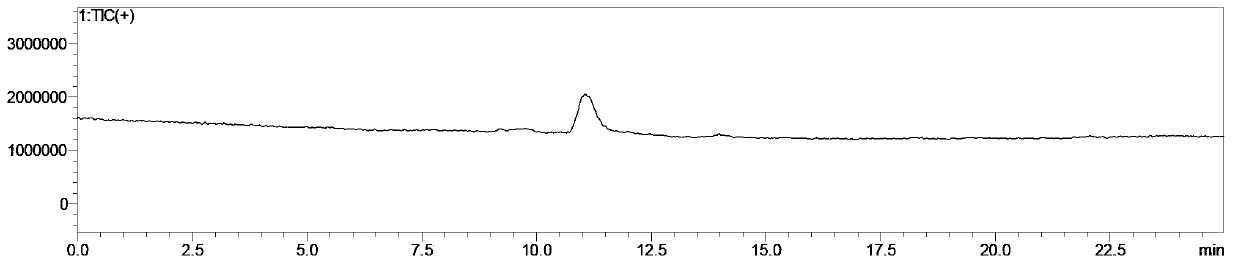
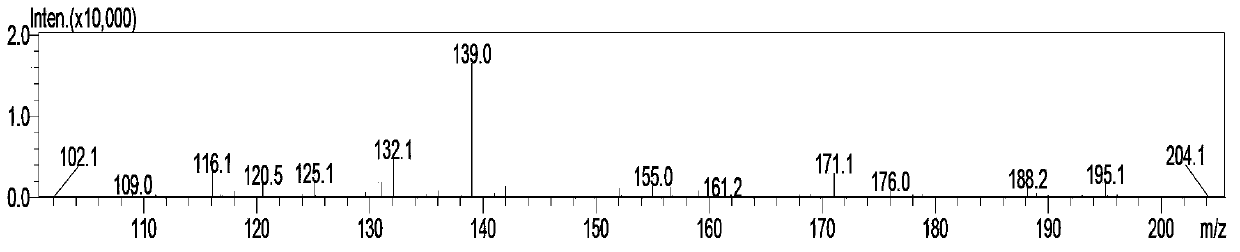
[0037] A simple and convenient preparation method of vitamin B1 key intermediate 2-methyl-4-amino-5-aminomethylpyrimidine, comprising the following steps:
[0038] (1) Add 3-formylaminopropionitrile 2 to the reactor, add organic solvent to dissolve; add catalytic amount of Lewis acid; The molar ratio of acetamidine to 3-formylaminopropionitrile is (1-2): 1, and the molar ratio of acetamidine to 3-formylaminopropionitrile (1.1-1.3): 1 is preferred; Detection of 3-formamidopropionitrile reaction is complete; then continue,
[0039] (2) Add anisole internal standard and orthoformate triester 5, the molar ratio of the amount of orthoformate triester to the 3-formamidopropionitrile used in step (1) is (2-3):1, React at a temperature of 65-105°C, and use the gas phase to detect that the reaction is complete; 2-methyl-4-formylamino-5-formylaminomethylpyrimidine 6 is obtained; then continue,
[0040] (3) Directly add an aqueous solution of inorganic alkali, hydrolyze at 80-100°C to ...
Embodiment approach
[0054] According to the present invention, a preferred embodiment is as follows:
[0055] A kind of convenient preparation method of vitamin B1 key intermediate 2-methyl-4-amino-5-aminomethylpyrimidine, the steps are as follows:
[0056] a. Add 104.5 grams of acetamidine hydrochloride and 220 grams of 27wt% sodium methoxide methanol solution in a 1000 ml glass reaction vessel, react at 10-20°C for 30 minutes, filter, and the filtrate is a methanol solution of acetamidine;
[0057] b. Add 98 grams of 3-formylaminopropionitrile, 520 grams of toluene, and 27.2 grams of anhydrous zinc chloride into a 1000 milliliter four-necked flask; heat, and the internal temperature rises to 75-80 ° C; dropwise add the above-mentioned Acetamidine alcohol solution; stirring reaction, the use of gas phase detection 3-formamidopropionitrile reaction is complete. Then add 1 gram of anisole internal standard and 254.4 grams of trimethyl orthoformate; react at 95 to 100 ° C, and use gas phase detect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com