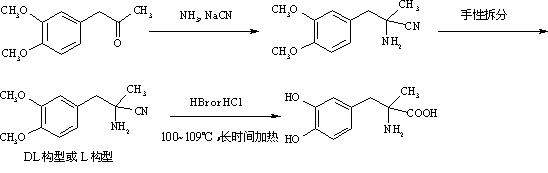
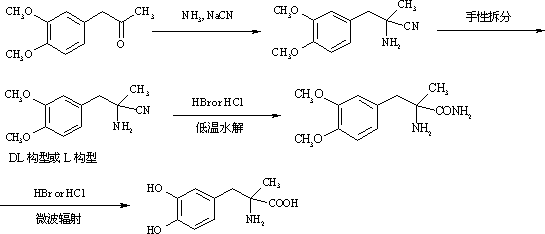
Method for preparing methyldopa from alpha-methyl-(3,4-dimethoxy phenyl)-alpha-aminopropionitrile by microwave hydrolysis method
A technology of dimethoxyphenyl and methyldopa, which is applied in the preparation of methyldopa by hydrolyzing α-methyl-(3,4-dimethoxyphenyl)-α-aminopropionitrile by a microwave hydrolysis method It can solve the problems of long reaction time, more acidic wastewater, and increased by-products, and achieve the effects of reducing energy and economic costs, reducing acid wastewater discharge, and improving purity and yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In a two-neck flask equipped with a thermometer and a stirrer, add 20.7 g (0.051 mol) of 9% concentrated hydrochloric acid. Cool to 0~5°C with ice water. Slowly add 2.18 g (0.0085mol) of DL-2-amino-3-(3,4-dimethoxyphenyl)-2-methyl-propionitrile hydrochloride [or L-2-amino-3-( 3,4-dimethoxyphenyl)-2-methyl-propionitrile hydrochloride, the same for the following examples] was dissolved in ice-cold concentrated hydrochloric acid. Stir vigorously for 1-2 hours after addition. Warm up to room temperature after the reaction is complete. The reaction mixture was placed in a chemical experiment temperature-controlled microwave instrument, and irradiated for 15 minutes at a microwave power of 250 watts. After the reaction was completed, the temperature was raised to room temperature, and N was directly introduced into the reaction mixture slowly. 2 , while extracting excess HCl by distillation under reduced pressure. Adjust the pH value of the solution with ammonia water an...
Embodiment 2
[0052] In a two-neck flask equipped with a thermometer and a stirrer, add 10.3 g (0.051 mol) of 18% concentrated hydrochloric acid. Cool to 0~5°C with ice water. Slowly add 2.18 g (0.0085mol) of DL-2-amino-3-(3,4-dimethoxyphenyl)-2-methyl-propionitrile hydrochloride [or L-2-amino-3-( 3,4-dimethoxyphenyl)-2-methyl-propionitrile hydrochloride, the same for the following examples] was dissolved in ice-cold concentrated hydrochloric acid. Stir vigorously for 1-2 hours after addition. Warm up to room temperature after the reaction is complete. The reaction mixture was placed in a chemical experiment temperature-controlled microwave instrument, and irradiated for 15 minutes at a microwave power of 250 watts. After the reaction was completed, the temperature was raised to room temperature, and N was directly introduced into the reaction mixture slowly. 2 , while extracting excess HCl by distillation under reduced pressure. Adjust the pH value of the solution with ammonia water a...
Embodiment 3
[0055] In a two-necked flask equipped with a thermometer and a stirrer, add 34.4 grams (0.051 mol) of 12% hydrobromic acid. Cool to 0~5°C with ice water. Slowly add 2.18 g (0.0085mol) of DL-2-amino-3-(3,4-dimethoxyphenyl)-2-methyl-propionitrile hydrochloride [or L-2-amino-3-( 3,4-dimethoxyphenyl)-2-methyl-propionitrile hydrochloride, the same for the following examples] was dissolved in ice-cold concentrated hydrochloric acid. Stir vigorously for 1-2 hours after addition. Warm up to room temperature after the reaction is complete. The reaction mixture was placed in a chemical experiment temperature-controlled microwave instrument, and irradiated for 15 minutes at a microwave power of 250 watts. After the reaction was completed, the temperature was raised to room temperature, and N was directly introduced into the reaction mixture slowly. 2, while extracting excess hydrobromic acid by distillation under reduced pressure. Adjust the pH value of the solution with ammonia wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com