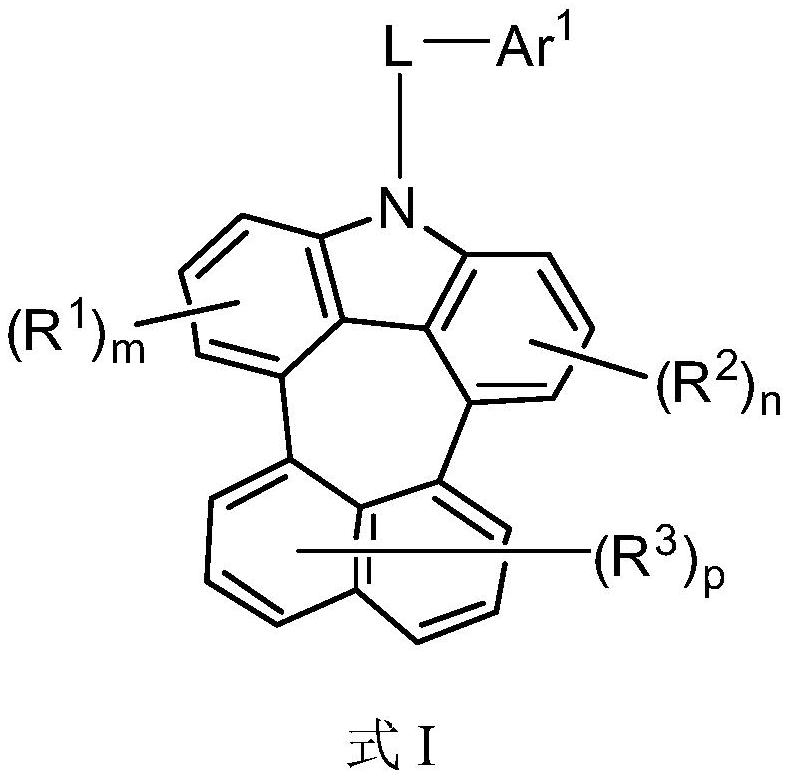
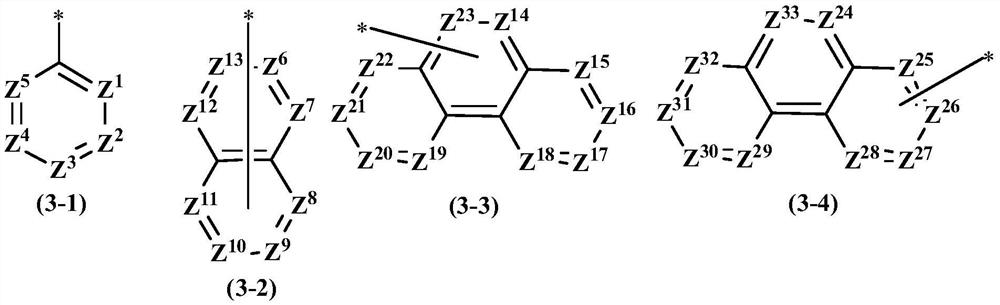
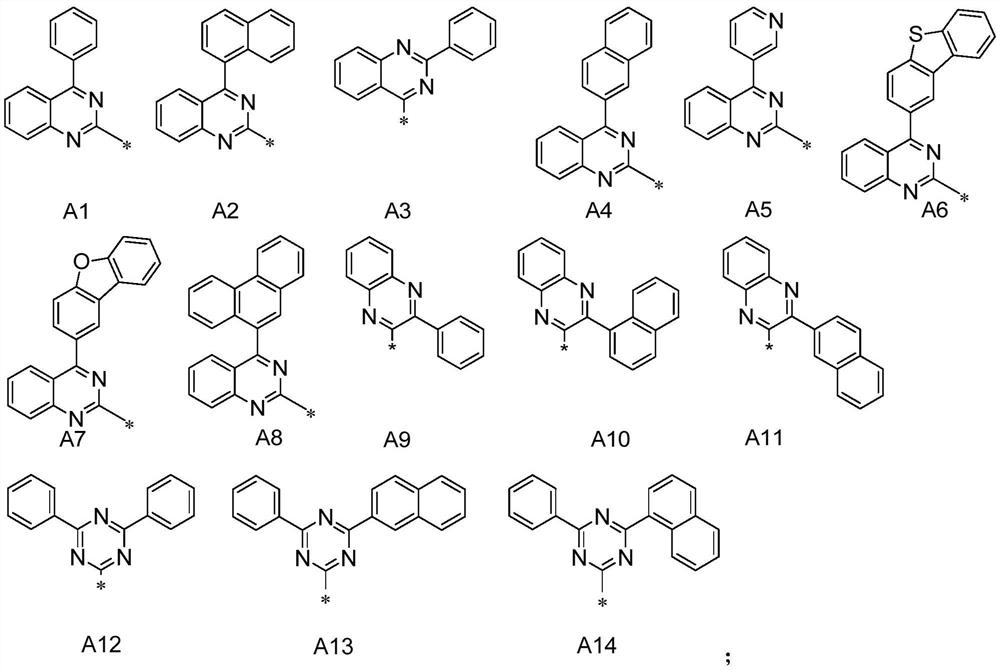
Compound and application thereof
A compound and unsubstituted technology, applied in the field of organic electroluminescence, can solve the problems of high transmission barriers, poor luminous efficiency and poor molecular structure stability, and achieve the effect of reducing transmission barriers and improving planarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0094] Synthesis Example 1: Synthesis of Intermediate E
[0095]
[0096] Add 1,8-dibromonaphthalene (1mol), phenylboronic acid (1mol), potassium carbonate (1.2mol), tetrakis(triphenylphosphine)palladium (0.01mmol), dioxane 1000mol, and water 100mL into the reaction flask , heated to reflux for 6h, the reaction was monitored by thin-layer chromatography (TLC), ethyl acetate and water were added for extraction, and the organic phase was concentrated to obtain Intermediate A.
[0097] Add A (0.7mol) to 1L of tetrahydrofuran (THF) and cool down to -78°C, add n-butyllithium (0.8mol) dropwise, keep warm for 30min, and then add triisopropyl borate (1mol) dropwise. After completion, gradually return to room temperature and react for 3 hours, add water and ethyl acetate for extraction, concentrate the organic phase, wash with methanol and filter to obtain Intermediate B.
[0098] Add B (0.5mol), 2-bromo-6-nitroaniline (0.7mol), potassium carbonate (1.0mol), tetrakis(triphenylphosp...
Synthetic example 2
[0101] Synthesis Example 2: Synthesis of Compound P7
[0102]
[0103] Add E (50mmol), 2-chloro-4-(1-naphthyl)quinazoline (55mmol), cesium carbonate (60mmol), N,N-dimethylformamide (DMF, 150mL) into the reaction flask, The reaction was refluxed for 8 hours. After the reaction was complete, it was lowered to room temperature. The reaction liquid was poured into water and filtered. The filter cake was washed once with ethanol and recrystallized from toluene to obtain compound P7.
Synthetic example 3
[0104] Synthesis Example 3: Synthesis of Compound P15
[0105]
[0106] 2,4 dichloroquinazoline (0.2mol), 4-(pyridin-3-yl)phenylboronic acid (1mol), potassium carbonate (0.35mol), tetrakis(triphenylphosphine)palladium (0.002mmol), dioxygen Add 300 mol of hexacyclic ring and 50 mL of water into the reaction flask, heat to 80°C for 4 hours, monitor the completion of the reaction by TLC, and filter to obtain P15-A after cooling.
[0107] Add E (50mmol), P15-A (55mmol), cesium carbonate (60mmol), DMF (150mL) into the reaction flask, reflux reaction for 8h, after the reaction is complete, cool down to room temperature, pour the reaction solution into water and filter, filter cake ethanol After washing once, toluene was recrystallized to obtain compound P15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com