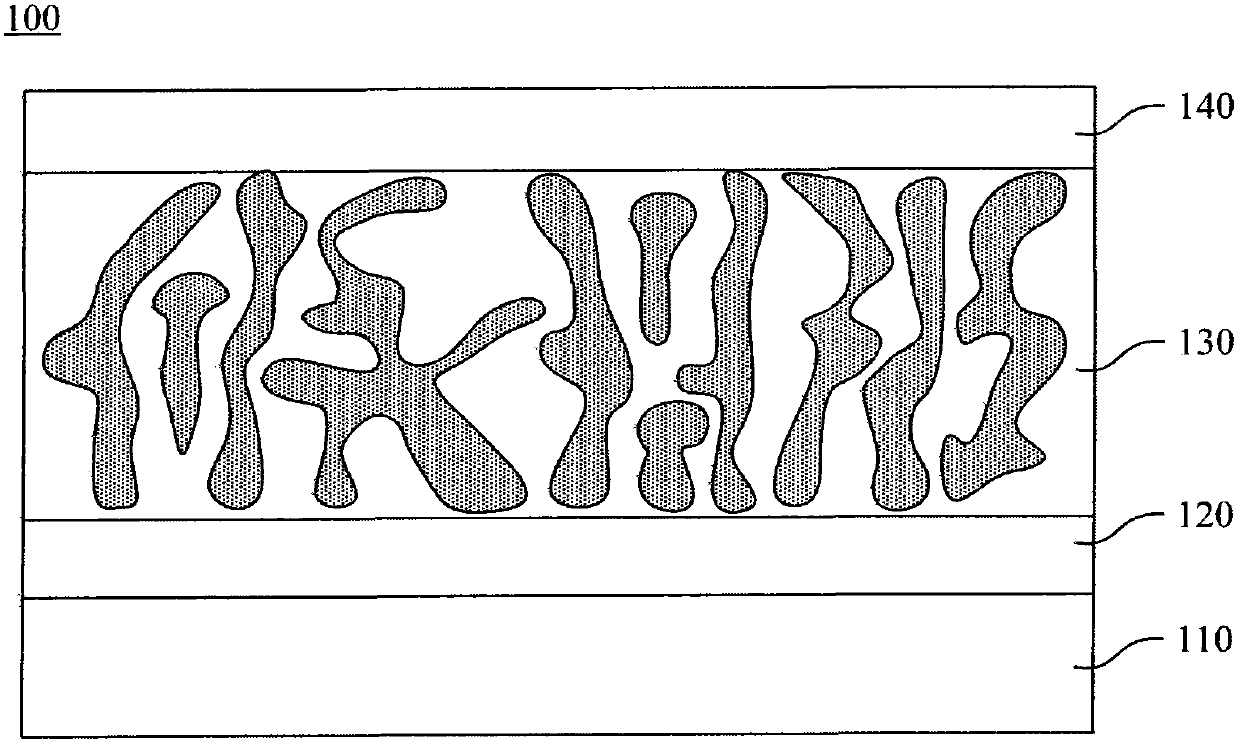
Organic semiconducting polymer and solar battery comprising same
An organic semiconductor and polymer technology, which can be used in semiconductor devices, semiconductor/solid-state device manufacturing, circuits, etc., and can solve problems such as the need to improve the conversion efficiency of organic solar cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Example 1: 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolin-2-yl)-9-(heptadecane-9-ylidene )-9H-fluorene preparation
[0139] Provided herein is 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9-(heptadecan-9-ylidene) -9H-fluorene (6) preparation method, its reaction process is shown in the chemical reaction formula (3).
[0140]
[0141] Chemical Reaction Formula (3)
[0142] (1) Preparation of 2,7-dibromo-9-[bis(methylsulfinyl)methylene]fluorene (2)
[0143] Put 6.48g (20mmol) of 2,7-dibromofluorene (1) into a 250mL three-necked flask, add 100mL of dimethyl sulfoxide, stir to dissolve and heat to 60°C. Then 6.4 g of sodium hydroxide (50% aqueous solution) was added, and the reaction was continued for 2 hours. It was then cooled to 0°C, and 2.2 mL of carbon disulfide was added. After reacting for 2 hours, 4 mL of methyl iodide was added and reacted overnight. Then the reaction solution was poured into ice water, 50 mL of ammonia water was added, suction fil...
Embodiment 2
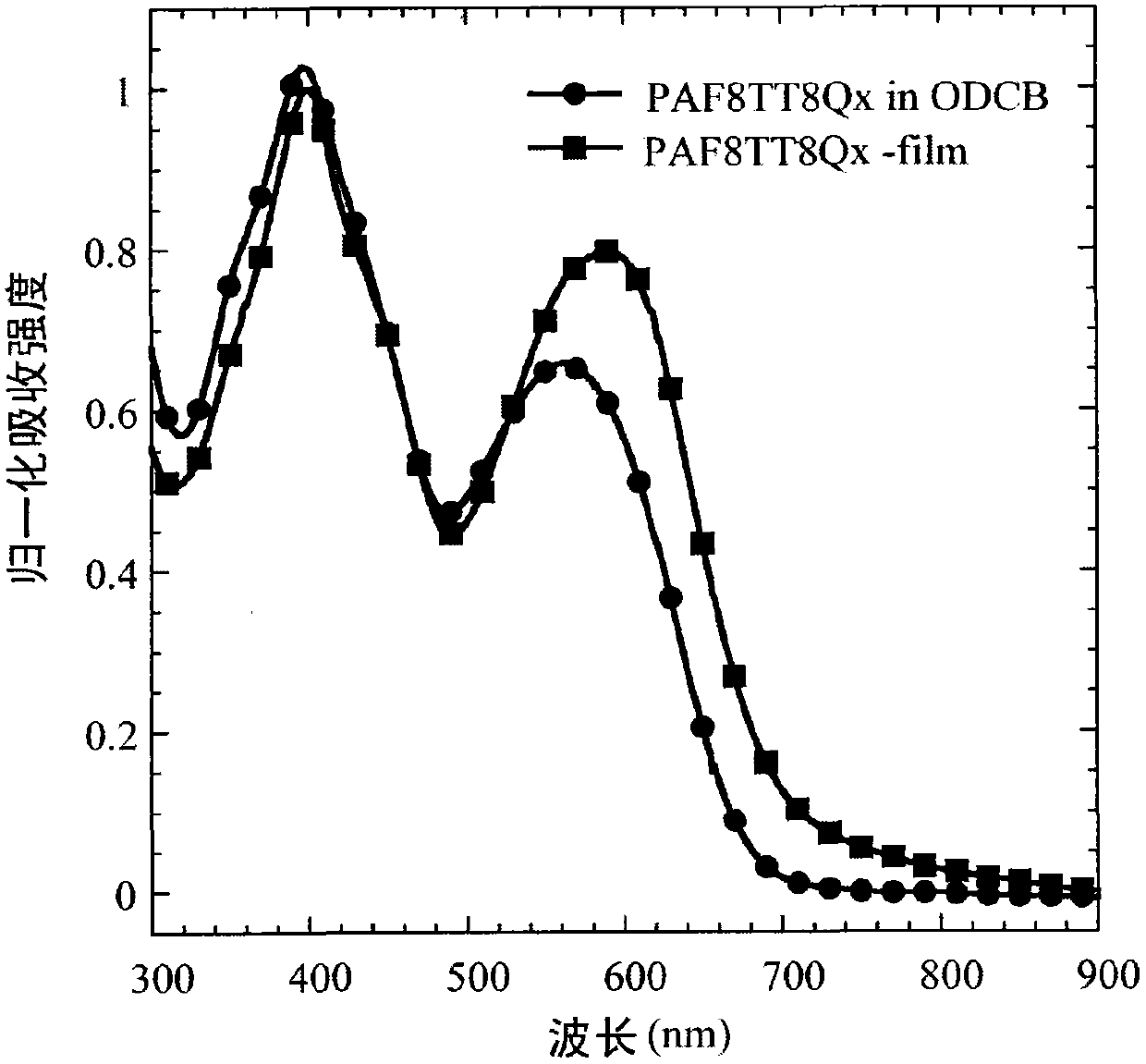
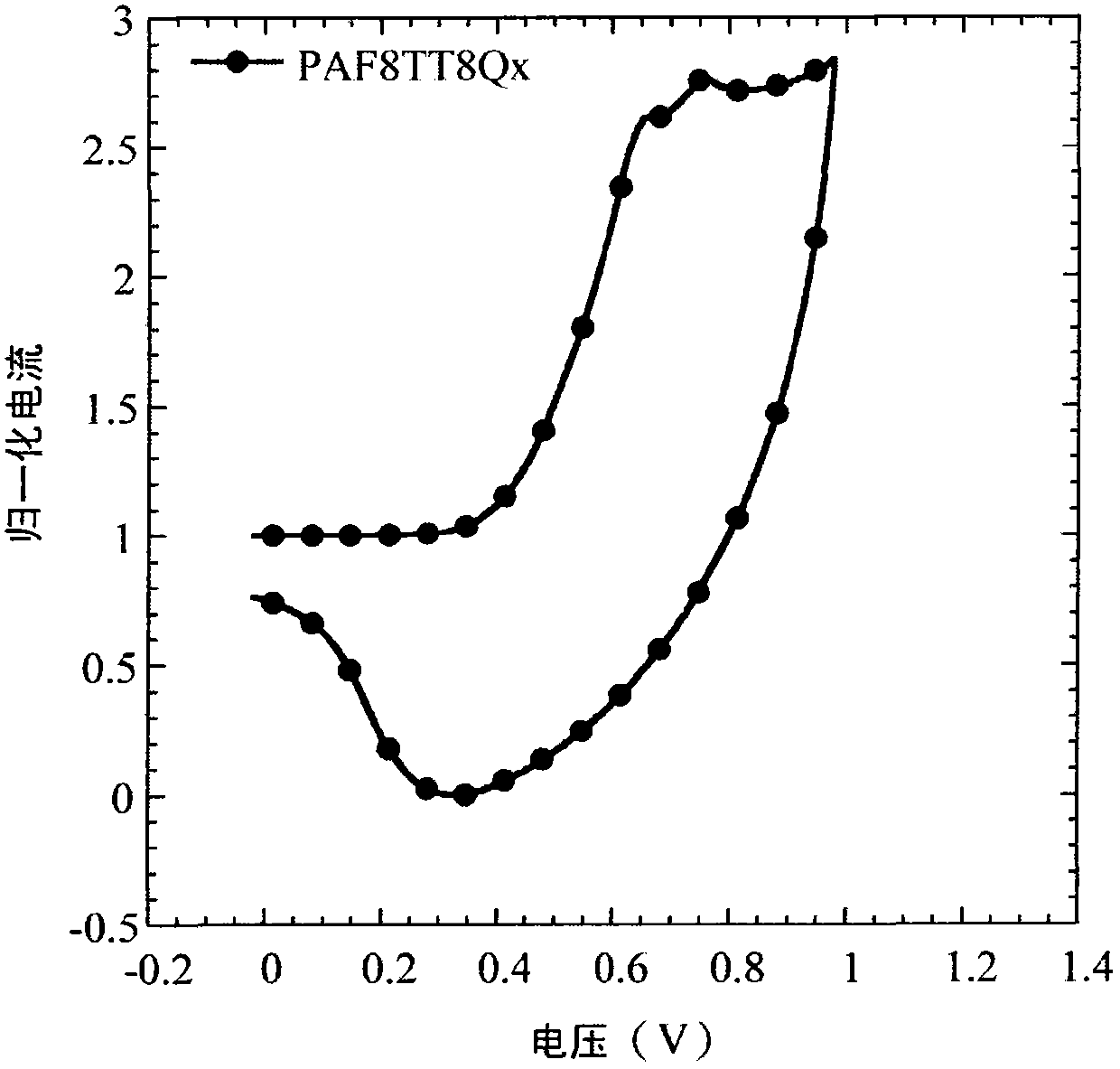
[0150] Example 2: Poly{9-(heptadecan-9-ylidene)-9H-fluorene-alternating-5,5-[5,8-dithiophen-2-yl-2,3-bis(5-octyl Preparation of thiophen-2-yl)quinoxaline]} (abbreviated as PAF8TT8Qx)
[0151] Provided herein is poly{9-(heptadecan-9-ylidene)-9H-fluorene-alternate-5,5-[5,8-dithiophen-2-yl-2,3-di(5-octyl The preparation method of thiophen-2-yl)quinoxaline]}(PAF8TT8Qx), the reaction process is shown in the chemical reaction formula (4).
[0152]
[0153] Chemical Reaction Formula (4)
[0154] 5,8-bis(5-bromothiophen-2-yl)-2,3-bis(5-octylthiophen-2-yl)quinoxaline (7) according to literature [Jie Zhang, Wanzhu Cai, Fei Huang, et al. Macromolecules, 2011, 44(4), pp 894-901] Preparation.
[0155] 164 mg (0.25 mmol) of the product (6) of Example 1, 210 mg (0.25 mmol) of 5,8-bis(5-bromothiophene-2-yl)-2,3-bis(5-octylthiophene-2- Base) quinoxaline (7), 9mg tris (dibenzylidene acetone) dipalladium (Pd 2 (dba)- 3 ) and 18 mg of tris(o-methyl)phenylphosphine (P(o-tol) 3 ) was plac...
Embodiment 3
[0158] Example 3: Poly{9-(heptadecan-9-ylidene)-9H-fluorene-alternating-5,5-[5,8-dithiophen-2-yl-2,3-bis(4-octyl Preparation of phenyl) quinoxaline]} (abbreviated as PAF8TP8Qx)
[0159] Provided herein is poly{9-(heptadecan-9-ylidene)-9H-fluorene-alternate-5,5-[5,8-dithiophen-2-yl-2,3-di(4-octyl The preparation method of phenyl) quinoxaline]} (PAF8TP8Qx), its reaction process is shown in chemical reaction formula (5).
[0160]
[0161] Chemical Reaction Formula (5)
[0162] 5,8-bis(5-bromothiophen-2-yl)-2,3-bis(4-octylphenyl)quinoxaline (8) according to literature [Wendimagegn Mammo, Shimelis Admassie, et al.Sol.Energy Mater.Sol.Cells.2007, 91(11), pp1010-1018] Preparation.
[0163] 164 mg (0.25 mmol) of the product (6) of Example 1, 207 mg (0.25 mmol) of 5,8-bis(5-bromothiophen-2-yl)-2,3-bis(4-octylbenzene-1- Base) quinoxaline (8), 9 mg of tris(dibenzylideneacetone) dipalladium and 18 mg of tris(o-methyl)phenylphosphine were placed in a 50 mL two-necked flask. Under t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com