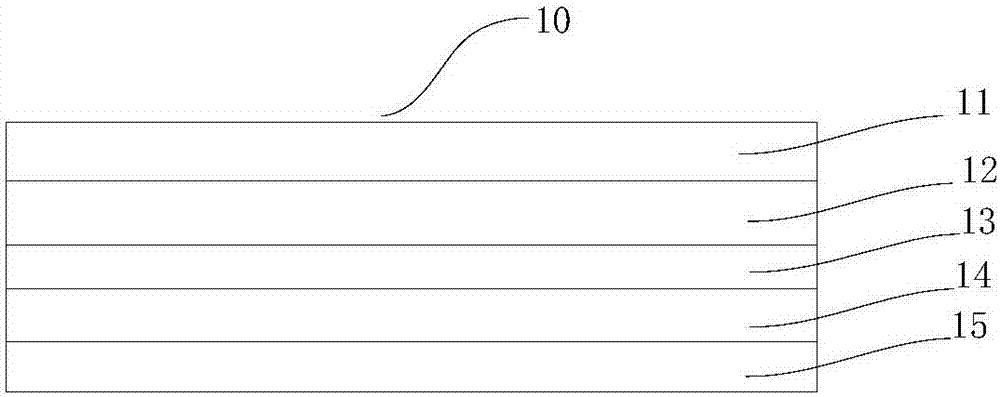
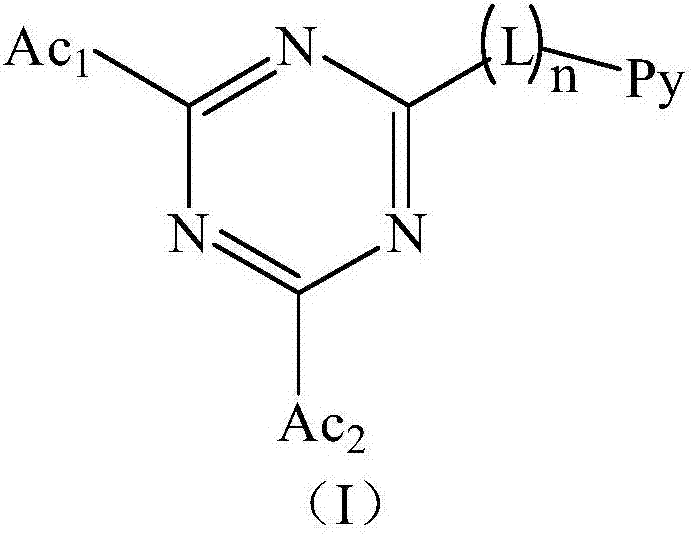
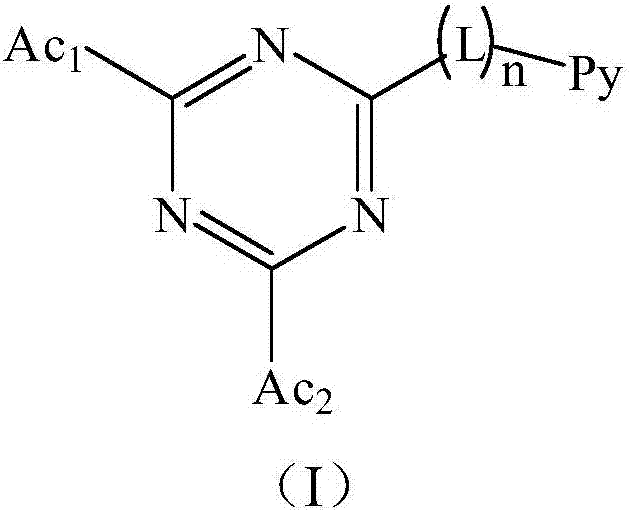
Triazine compound and light-emitting device
A triazine compound, fused technology, applied in the field of triazine compounds and light-emitting devices, can solve problems such as unfavorable charge balance and carrier transport, hole transport performance and large electron transport performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0090] 1. Synthesis of Compound C-1
[0091]
[0092] Dissolve 2mmol of the raw material (C-1-1) in 25mL of tetrahydrofuran, add dropwise a butyllithium solution containing 2mmol of butyllithium under nitrogen protection, and stir for 10 minutes, then add the solvent dropwise to Triazine in 25 mL THF solution. After stirring for 10 minutes, the temperature was raised to reflux for 6 hours. 100 mL of water was added to precipitate a solid, and the solid was washed with water and n-hexane in turn. Recrystallization from ethanol gave intermediate (C-1-2). Get intermediate (C-1-2) 1mmol, aromatic boronic acid compound (C-1-3) 1.2mmol, 0.1g tetrakis (triphenylphosphine palladium), 3mL concentration is the sodium carbonate solution of 2mol / L, 4mL toluene and 2mL of ethanol was heated and stirred at 80°C for 8 hours under nitrogen protection. The reaction solution was extracted with dichloromethane, washed with water, dried over anhydrous sodium sulfate, and distilled under re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com