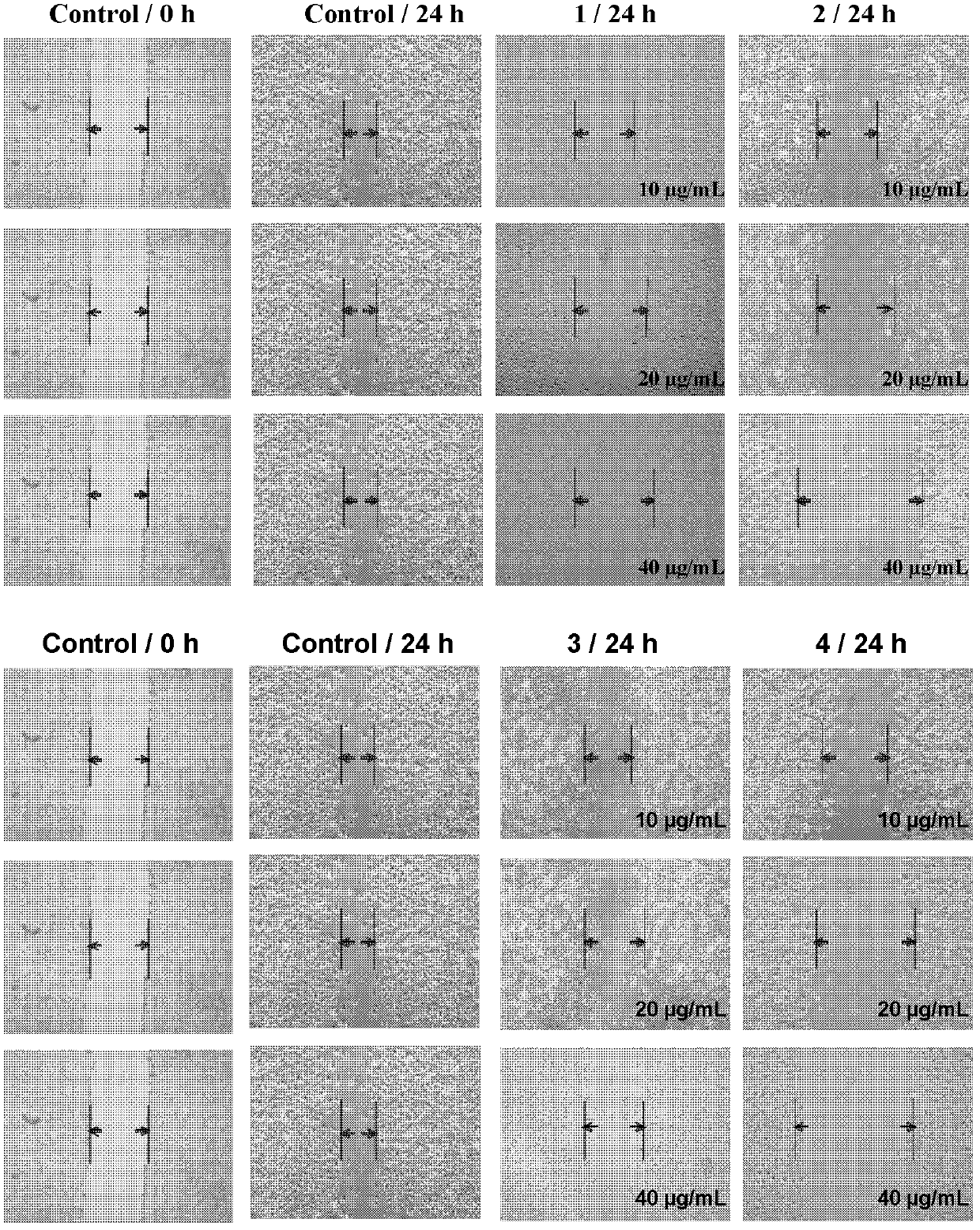
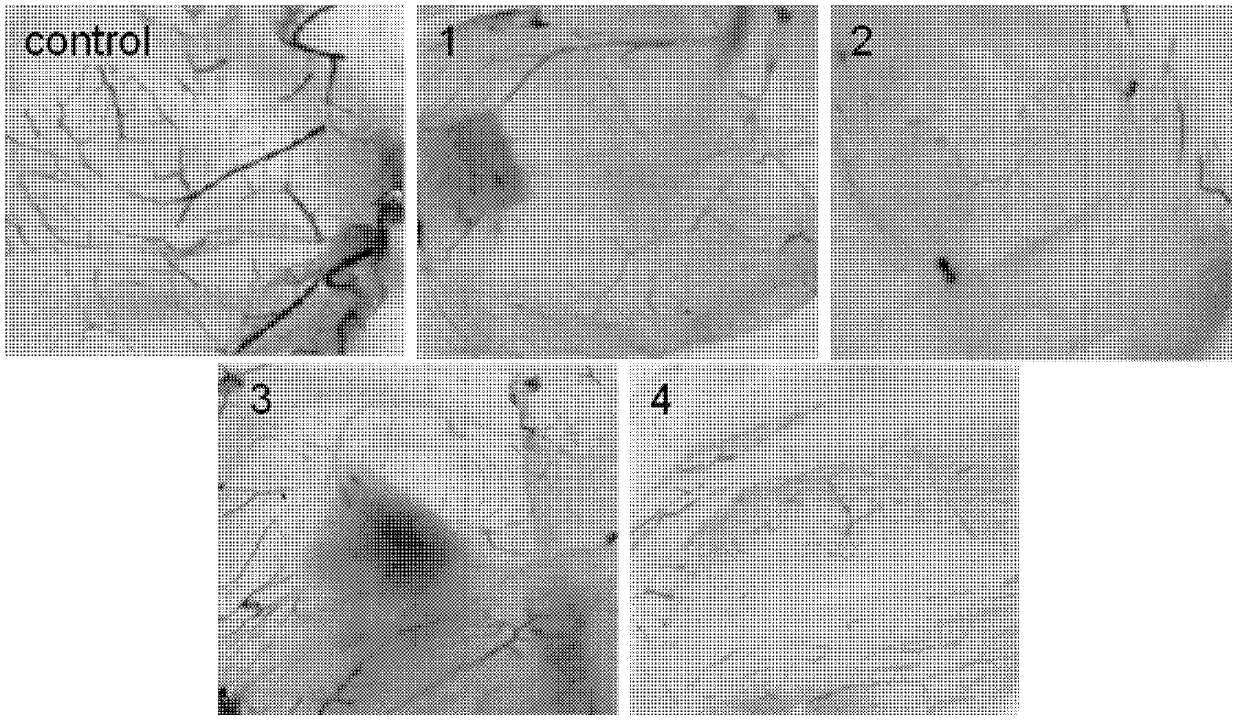
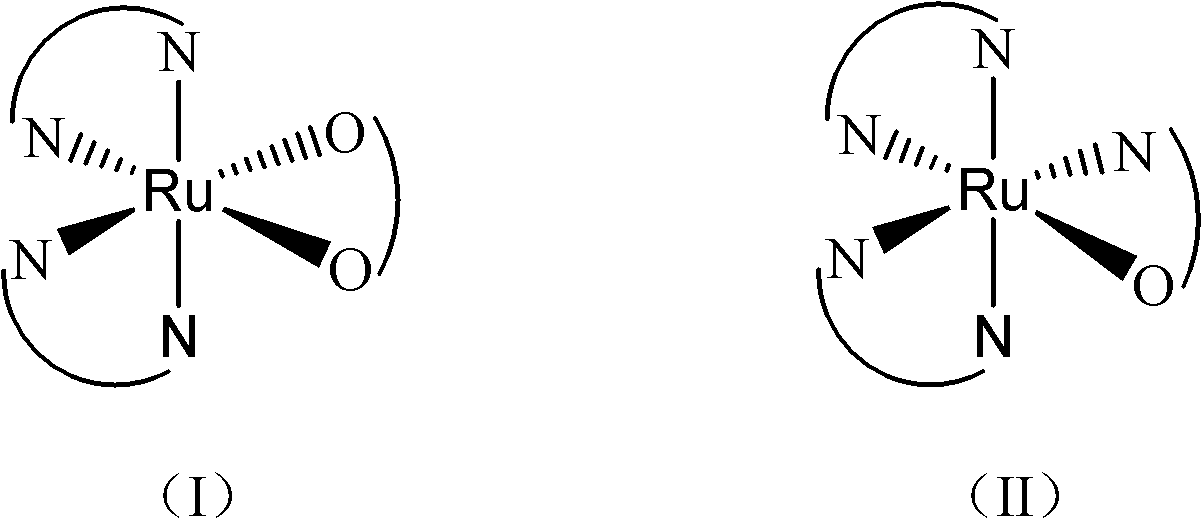
Ruthenium complex capable of inhibiting tumor angiogenesis and preparation method and application thereof
A technology of ruthenium complexes and tumor blood vessels, which is applied in the field of ruthenium complexes that inhibit tumor blood vessel formation and its preparation, can solve problems such as poor stability, difficult hydrolysis, and high toxicity, and achieve good stability, difficult hydrolysis, and reduced toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Complex a: Ru(bpy) 2 Preparation of (ox) (wherein R is H)
[0073] (1) Synthesis of silver oxalate
[0074] Aqueous silver nitrate solution (11 mmol) was added dropwise to sodium oxalate solution (5 mmol), immediately forming a white precipitate. The reaction was stirred for 10 minutes and filtered. The white precipitate was washed with water and dried in vacuum to obtain silver oxalate (yield: 80%).
[0075] (2)cis-[Ru(bpy) 2 Cl 2 ]·2H 2 Synthesis of O
[0076] RuCl 3 ·nH 2 O (1.56g, 6mmol), bipyridine (1.87g, 12mmol) and lithium chloride (1.68g, 28mmol) were placed in a three-necked flask, 10mL of DMF was added, and heated to reflux for 8 hours under the protection of argon. After cooling to room temperature, 50 mL of acetone was added, and the mixture was kept overnight at 4°C. Suction filtration, precipitation with ice water, washing several times with acetone at 4°C, and drying in vacuo to obtain 2.52 g of purple-black microcrystals, namely cis-...
Embodiment 2
[0081] Example 2 Complex d: Ru(phen) 2 (mal) (where R 1 For the preparation of H)
[0082] (1) Synthesis of silver malonate
[0083] Aqueous silver nitrate solution (11 mmol) was added dropwise to sodium malonate solution (5 mmol), immediately forming a white precipitate. The reaction was stirred for 10 minutes and filtered. The white precipitate was washed with water and dried in vacuo to obtain silver malonate (yield: 78%).
[0084] (2)cis-[Ru(phen) 2 Cl 2 ]·2H 2 Synthesis of O
[0085] called RuCl 3 ·nH 2 O 1.56g (6mmol), with o-phenanthroline 2.40g (12mmol) and lithium chloride 1.68g (28mmol), dissolved in 10mL DMF, heated to reflux for 8h under the protection of argon. After cooling to room temperature, 50 mL of acetone was added, and it was kept overnight at 4°C. Suction filtration, precipitation with ice water, washing with acetone at 4°C, and vacuum drying to obtain purple-black microcrystals, namely cis-[Ru(phen) 2 Cl 2 ]·2H 2 O, yield 72%.
[0086] (3)...
Embodiment 3
[0090] Example 3 Complex f: Ru(bpy) 2 (mal) (where R, R 1 For the preparation of H)
[0091] (1) Synthesis of silver malonate
[0092] With embodiment 2 step (1).
[0093] (2)cis-[Ru(bpy) 2 Cl 2 ]·2H 2 Synthesis of O
[0094] With embodiment 1 step (2).
[0095] (3)Ru(bpy) 2 Synthesis of (mal)
[0096] cis-[Ru(bpy) 2 Cl 2 ]·2H 2 O (0.5 mmol) and silver malonate (0.7 mmol) were reacted at 60° C. for 12 hours in a mixed solvent of ethanol / water (V:V=1:3). The mixture was filtered through celite to remove the precipitated silver chloride. The solvent was spun off under vacuum and the solid was dissolved with methanol. The solvent was reduced to about 5% of the original capacity, diethyl ether was added, overnight at -20°C, the precipitate was filtered and recrystallized with methanol-diethyl ether (V:V=1:1).
[0097] The structural characterization data of the final product are as follows: Found: C: 53.61; H; 3.51; N; 10.85.(Calc.forC 23 h 18 N 4 o 4 Ru: C: 53....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com