A kind of polysubstituted furanocycloheptatriene pyrrole derivative and preparation method thereof
A technology of cycloheptatrienylpyrrole and multi-substituted furan, which is applied in the field of multi-substituted furocycloheptatrienylpyrrole derivatives and their preparation, can solve the problems of low atom utilization, complex process, and harsh reaction conditions, etc., and achieve The raw materials and reagents are stable and easy to obtain, the preparation method is simple, and the water and oxygen tolerance is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
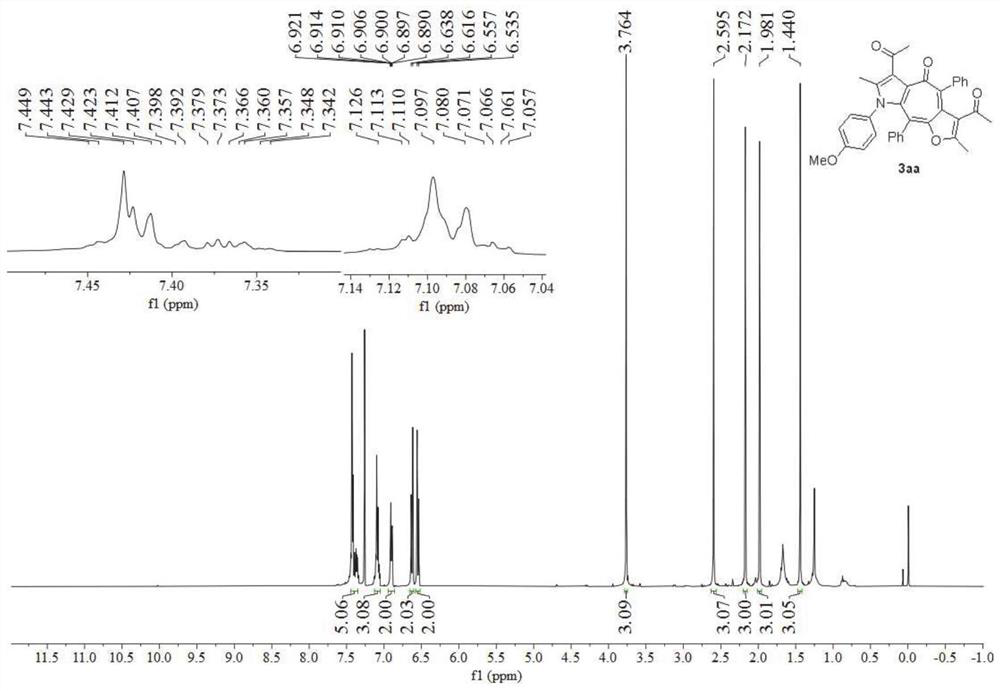
[0049] Preparation of polysubstituted furanocycloheptatriene pyrrole derivatives 3aa:
[0050]
[0051] To a 15mL pressure tube, add p-methoxyphenylisonitrile 1a (0.3mmol), 3-(3-phenylpropyl-2-yn-1-ylidene)-2,4-pentanedione 2a (0.75 mmol), and was dissolved in dichloromethane (2 mL), a stirring bar was added, zinc chloride (0.3 mmol) was added, and after stirring at room temperature for 10 minutes, m-chloroperoxybenzoic acid (0.3 mmol) was added to the reaction system, Continue to stir the reaction system at room temperature for 18h, at which time TLC monitors the complete formation of 3aa, then add aqueous sodium bisulfite solution (0.1N×5mL) to the system to quench the reaction, and then use dichloromethane (10mL×3) Extract the water phase, mix the obtained organic phases, add anhydrous sodium sulfate to dry, then evaporate the organic solvent by a rotary evaporator, and finally, separate by silica gel column chromatography to obtain the final product. Detection confirme...
Embodiment 2
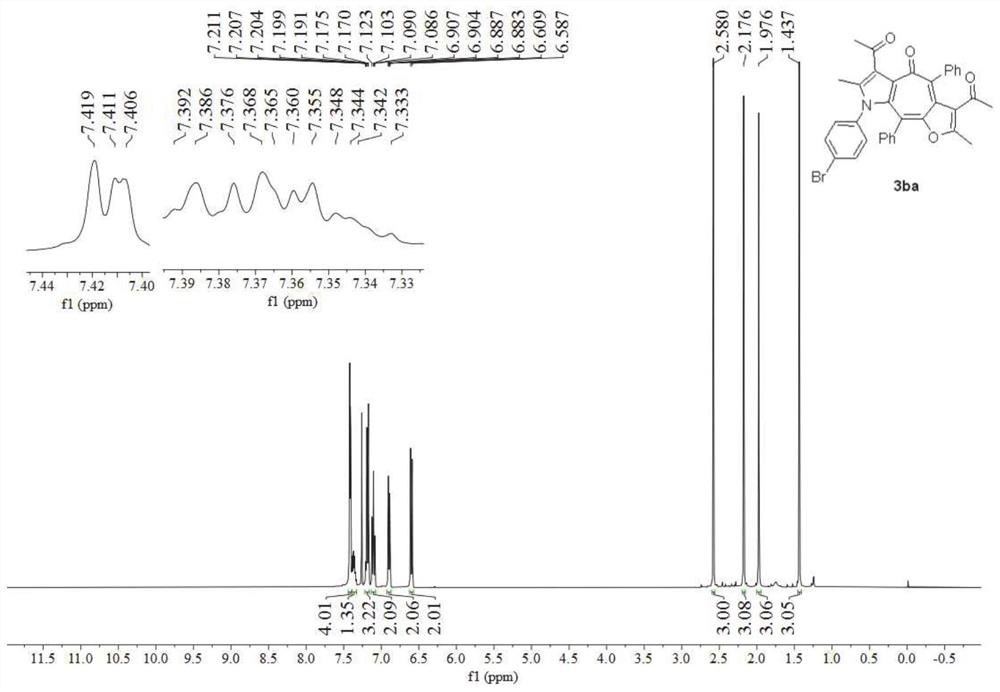
[0055] Preparation of polysubstituted furanocycloheptatriene pyrrole derivatives 3ba:
[0056]
[0057] Using p-bromophenylisonitrile 1b instead of p-methoxyphenylisonitrile 1a in Example 1, other conditions were the same as in Example 1, to obtain a polysubstituted furanocycloheptatriene pyrrole derivative 3ba with a yield of 74% .
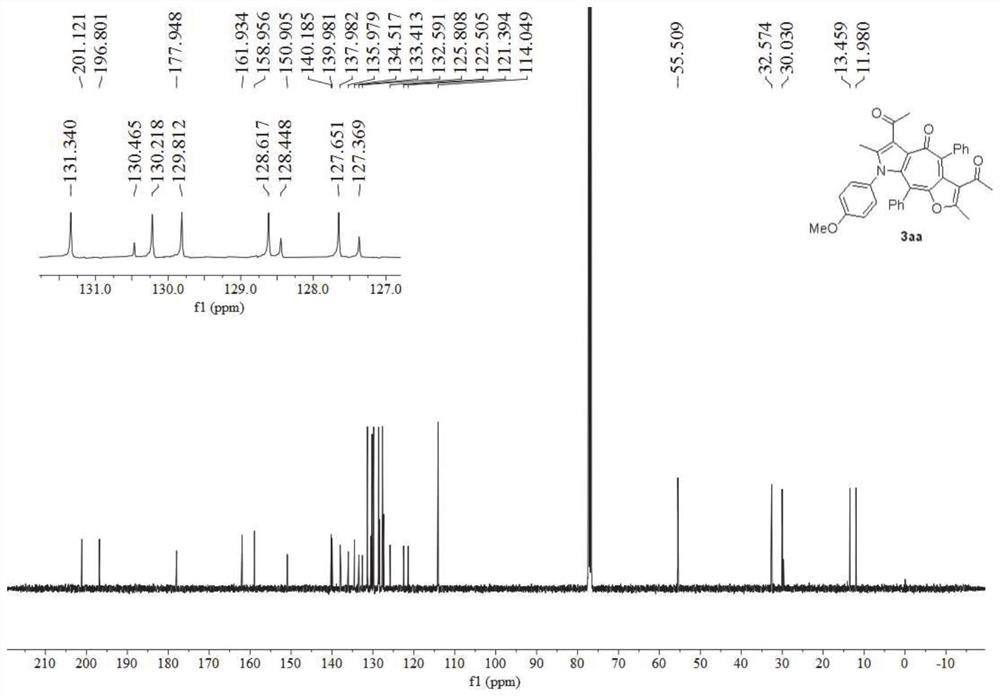
[0058] image 3 is the hydrogen nuclear magnetic spectrum of the furanocycloheptatriene pyrrole derivative obtained in Example 2 of the present invention, Figure 4 For its carbon NMR spectrum, spectrum analysis data:
[0059] 1 H NMR (400MHz, CDCl 3 )δ7.44-7.39(m,4H),7.39-7.33(m,1H),7.22-7.16(m,3H),7.10(t,J=7.5Hz,2H),6.92-6.87(m,2H) ,6.60(d,J=8.6Hz,2H),2.58(s,3H),2.18(s,3H),1.98(s,3H),1.44(s,3H). 13 C NMR (101MHz, CDCl 3 )δ200.8,196.6,177.8,162.0,150.9,139.8,139.3,138.2,136.8,136.1,134.2,133.3,132.7,131.9,131.2,130.4,130.3,128.6,128.5,127.8,127.6,126.2,122.5,122.3,120.8 ,32.5,30.0,13.4,11.9.HRMS(ESI-TOF)m / z calculated for C 35 H 26 ...
Embodiment 3
[0061] Preparation of polysubstituted furanocycloheptatriene pyrrole derivatives 3ca:
[0062]
[0063] Using p-ethoxycarbonyl phenyl isonitrile 1c instead of p-methoxyphenyl isonitrile 1a in Example 1, other conditions are the same as in Example 1, to obtain a polysubstituted furanocycloheptatriene pyrrole derivative 3ca with a yield of 72%.
[0064] Spectrogram analysis data:
[0065] 1 H NMR (400MHz, CDCl 3 )δ7.74(d, J=8.4Hz, 2H), 7.43–7.33 (m, 5H), 7.12–7.06 (m, 1H), 7.05–7.00 (m, 2H), 6.94–6.88 (m, 2H) ,6.81(d,J=8.4Hz,2H),4.38(q,J=7.1Hz,2H),2.58(s,3H),2.18(s,3H),1.95(s,3H),1.44(s, 3H),1.40(t,J=7.1Hz,3H). 13 C NMR (101MHz, CDCl 3 )δ200.7,196.6,177.9,165.3,162.1,150.9,141.7,139.8,139.2,138.3,136.1,134.1,133.3,132.9,131.2,130.5,130.0,129.9,128.8,128.6,128.5,127.8,127.7,126.3,122.5 ,120.8,61.3,32.5,30.0,14.2,13.4,11.9.HRMS(ESI-TOF)m / zcalculated for C 38 H 31 NNaO 6 + ([M+Na] + ) 620.2044, found 620.2023.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com