Methods of treating acute stress disorder and posttraumatic stress disorder
A post-traumatic stress and trauma technology, applied in the direction of pharmaceutical formulations, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of ASD or PTSD that are difficult to achieve and drugs are ineffective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0339] Example 1. Cyclobenzaprine Sublingual Formulation TNX-102SL
[0340] TNX-102SL is a sublingual formulation containing a eutectic melt of cyclobenzaprine hydrochloride (active ingredient) and D-mannitol. The formulation also contains a dibasic potassium salt. Table 1 shows the specific composition of TNX-102SL tablets.
[0341] Table 1: TNX-102SL Sublingual Tablet Composition
[0342]
[0343] a Mannitol: about 0.7 mg of the 2.5 mg total amount is a eutectic component, and the remainder is diluent.
[0344] b Flash is the trade name for an excipient containing approximately 80% mannitol and 20% cornstarch.
[0345] c Calculated as HCl salt
Embodiment 2
[0346] Example 2. Efficacy of TNX-102SL for the treatment of PTSD
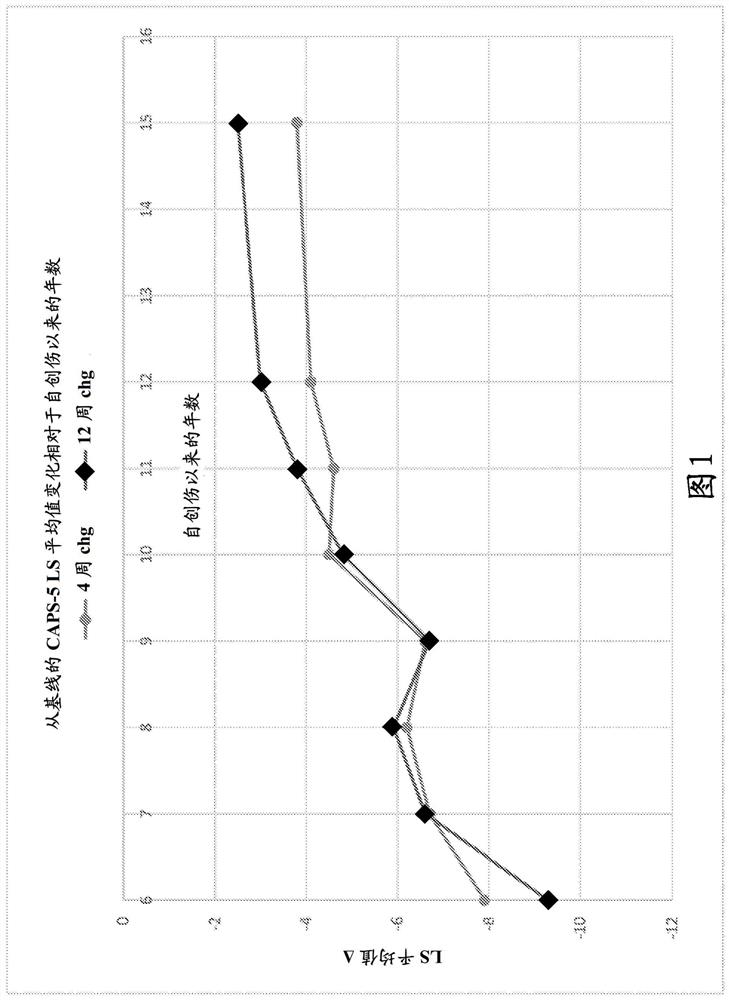
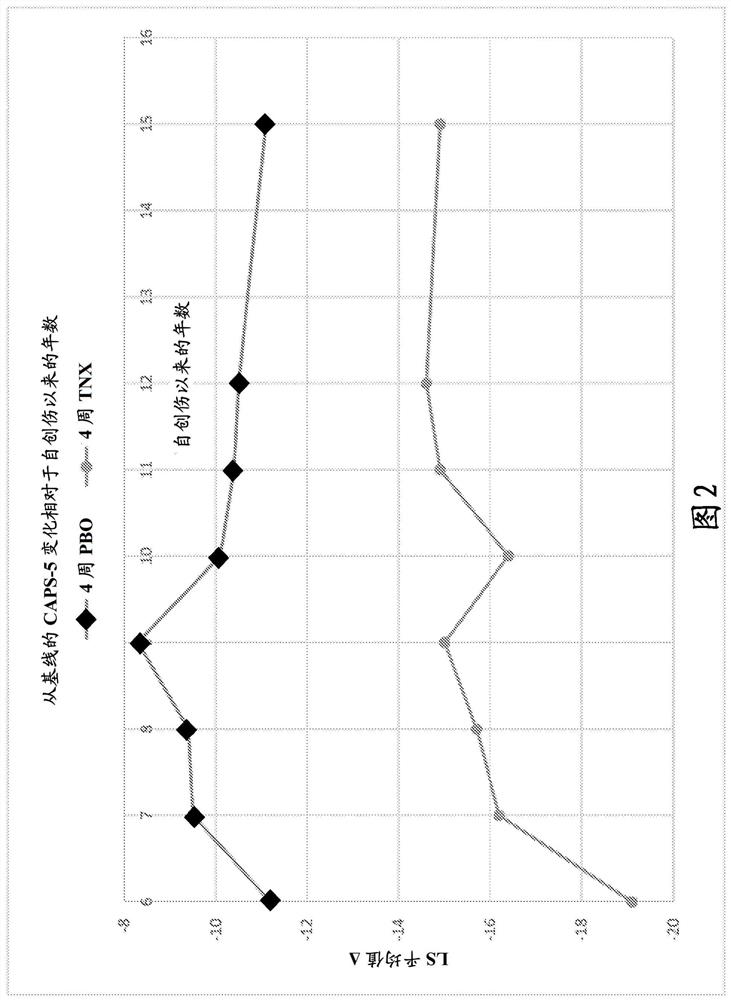
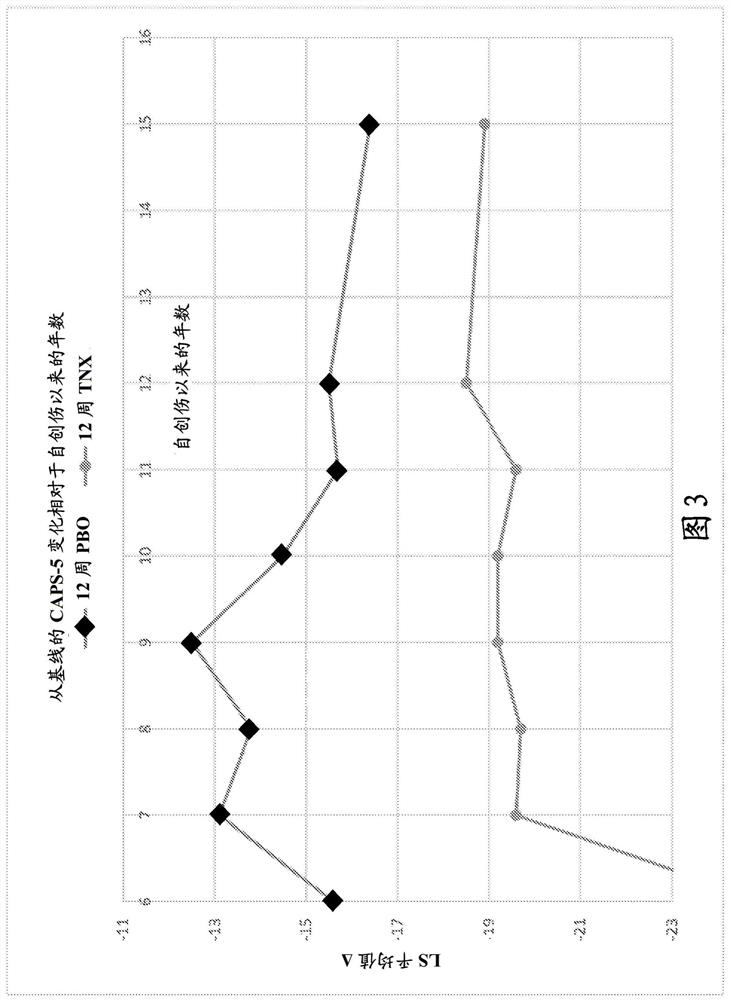
[0347] Two 12-week multicenter, randomized, double-blind, placebo-controlled, fixed-dose trials (P201 and P301) were conducted to investigate the efficacy and safety of a sublingual formulation of cyclobenzaprine (TNX-102SL). Both trials required PTSD DSM-5 Criterion A trauma occurring during military service since 2001; no antidepressants for ≥ 2 months; no or washed off other psychotropic medications. Both ruled out serious risk of suicide (intention or planning; attempt within one year); substance use disorder (SUD) within 6 months; lifetime of bipolar disorder, psychotic disorder, OCD, or antisocial personality disorder.
[0348] The trial analyzed the change from baseline in the severity of PTSD symptoms over the course of 12 weeks of treatment by subjects treated with a sublingual cyclobenzaprine formulation (TNX-102SL, 5.6 mg) and those receiving placebo. Between-subjects as measured by the DSM-5 Clini...
Embodiment 3
[0363] Cyclobenzaprine metabolism in subjects with a history of smoking
[0364] Determining a therapeutic dose of cyclobenzaprine, amitriptyline, or a pharmaceutically acceptable salt thereof is important to the overall efficacy of the treatment. Therapeutic dosage may be affected by various factors, including the subject's history of smoking and other drug use. In one aspect of the disclosure, subjects with a history of smoking have an attenuated response to treatment with TNX-102SL. Such as Figure 5 Depicted, after 4 weeks of treatment, subjects with a history of smoking (bottom) had a reduction in CAPS-5 scores relative to subjects receiving placebo. However, this was to a lesser extent than for subjects with no history of smoking (top). Furthermore, after 8 and 12 weeks of treatment, respectively, the subjects' response to the treatment eventually leveled off relative to the placebo. Smoking is known to be a strong inducer of CYP1A2. Without wishing to be bound by t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com