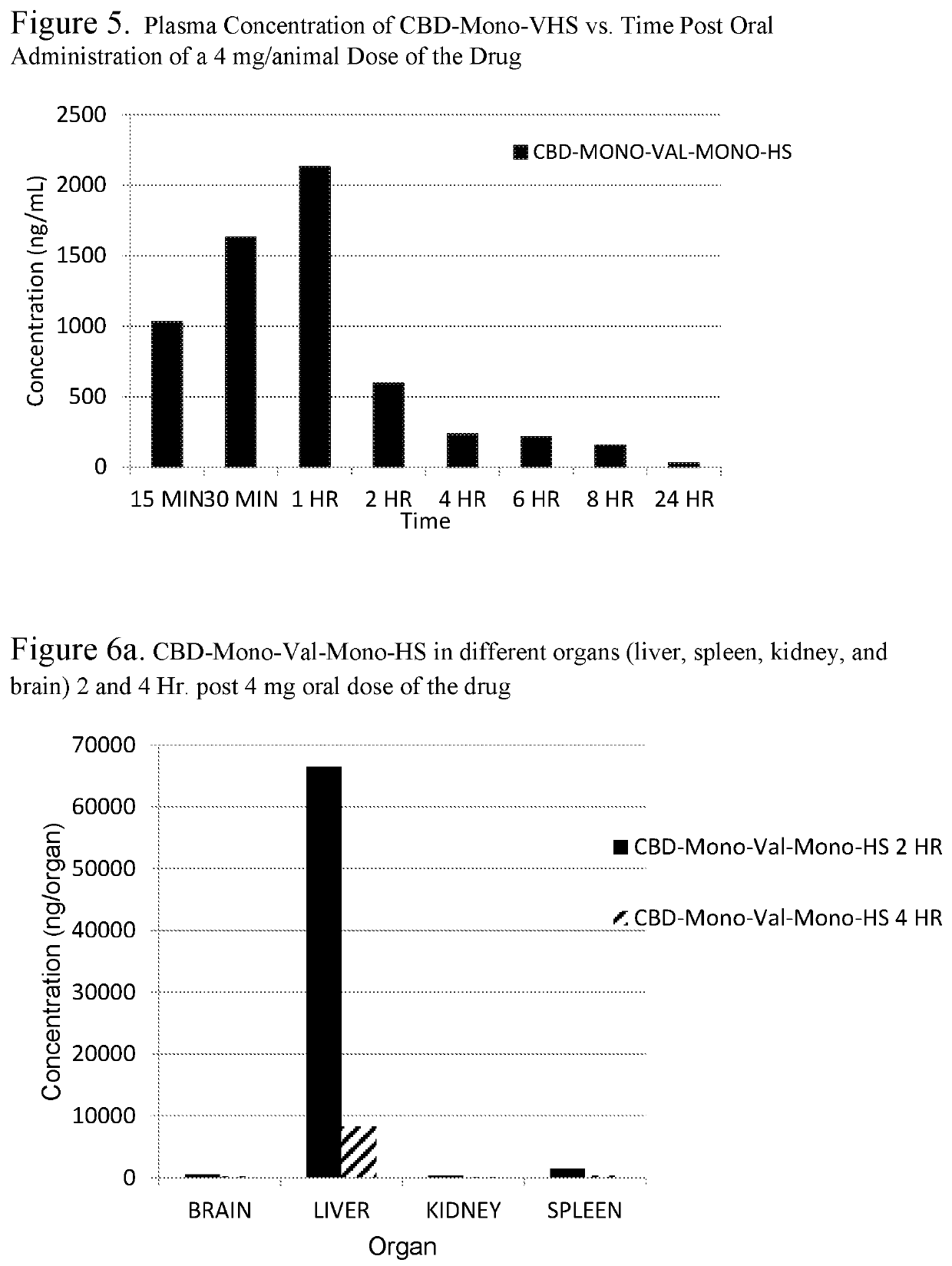
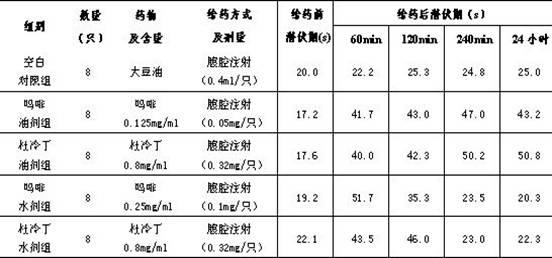
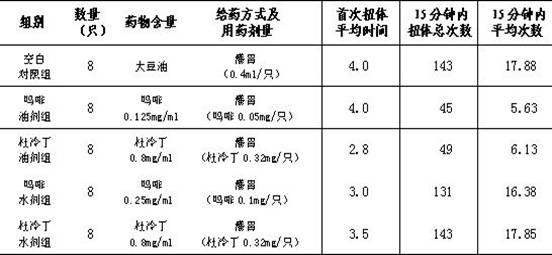
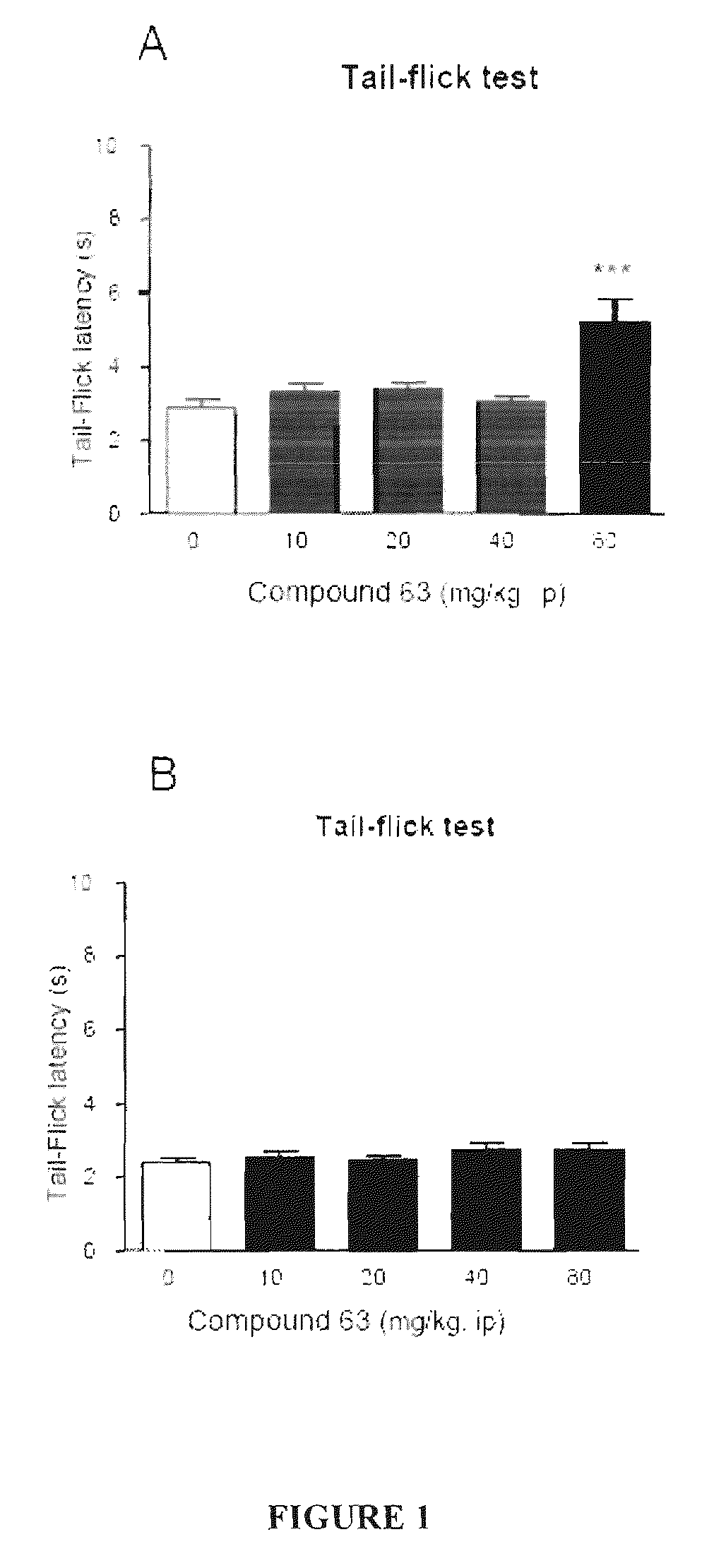
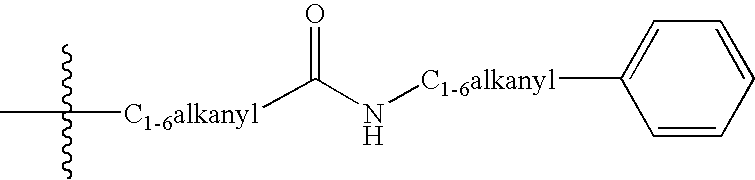
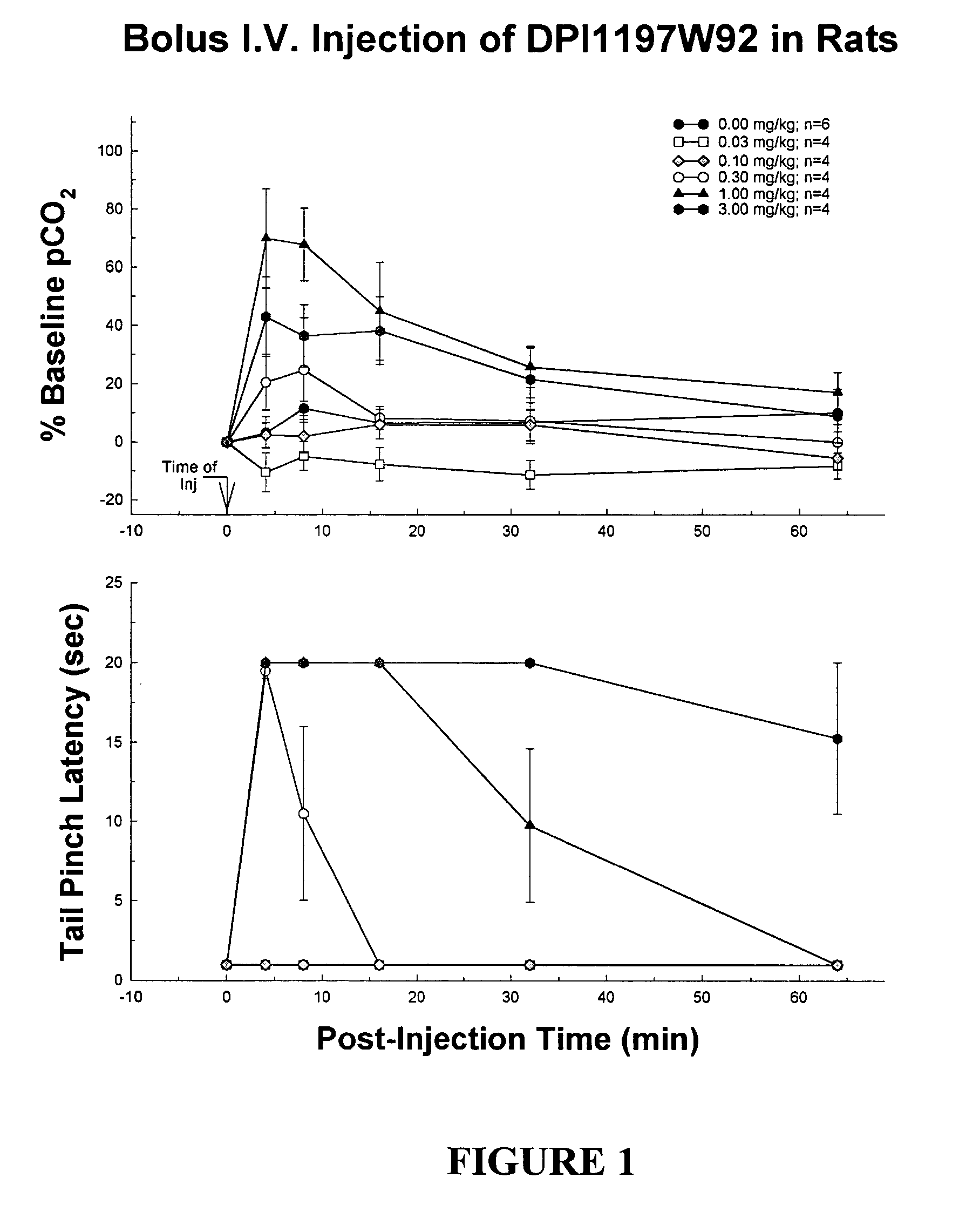
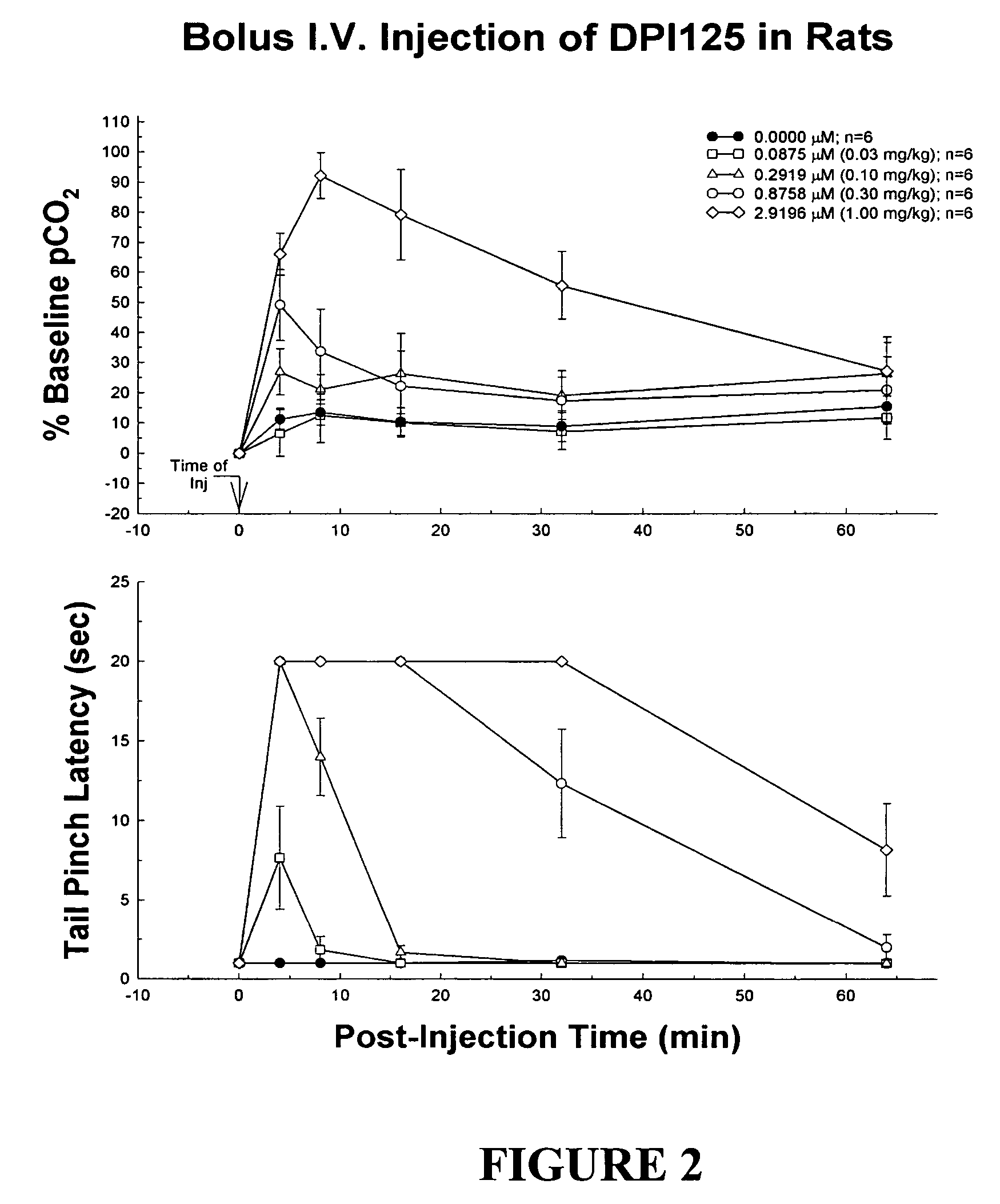
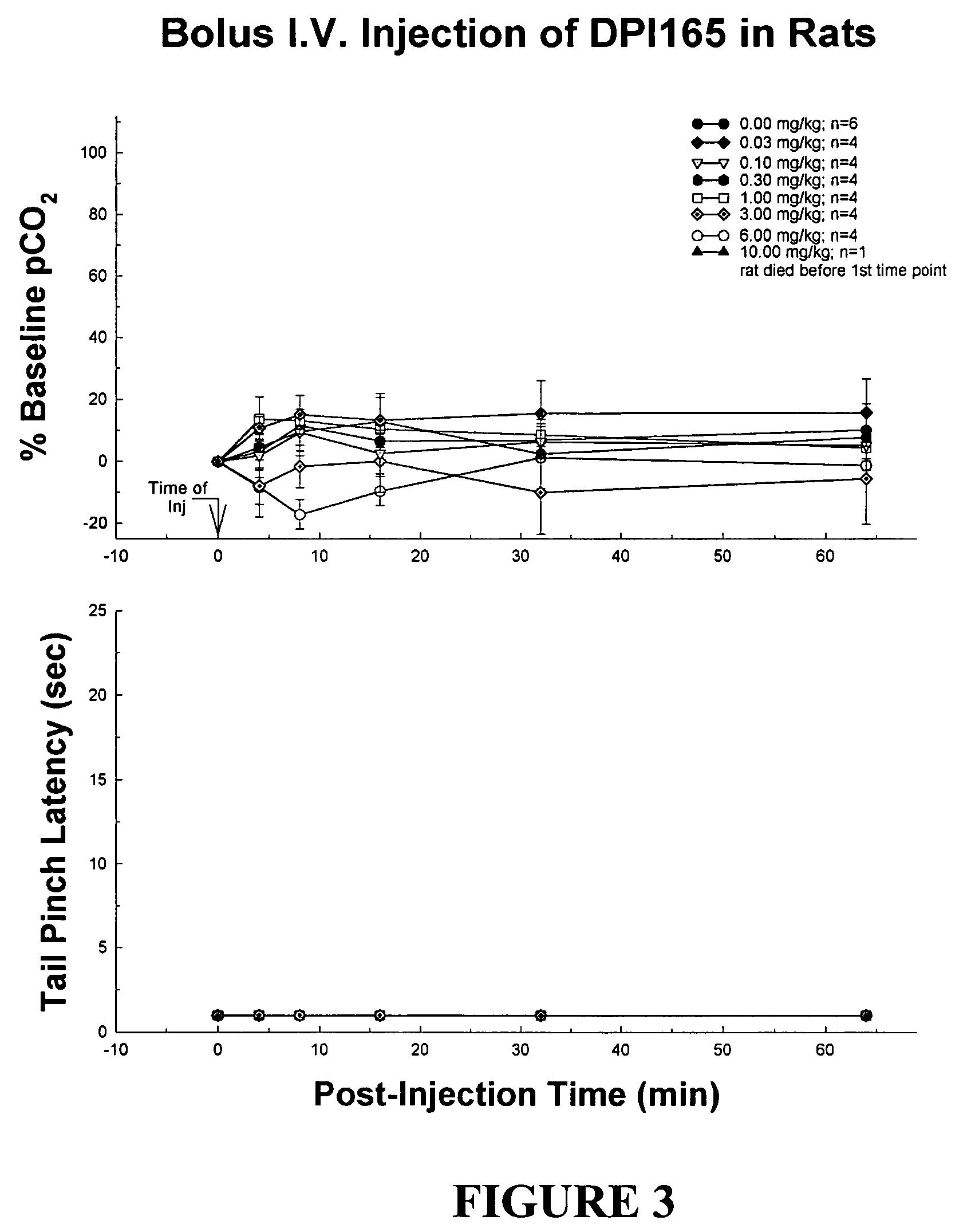
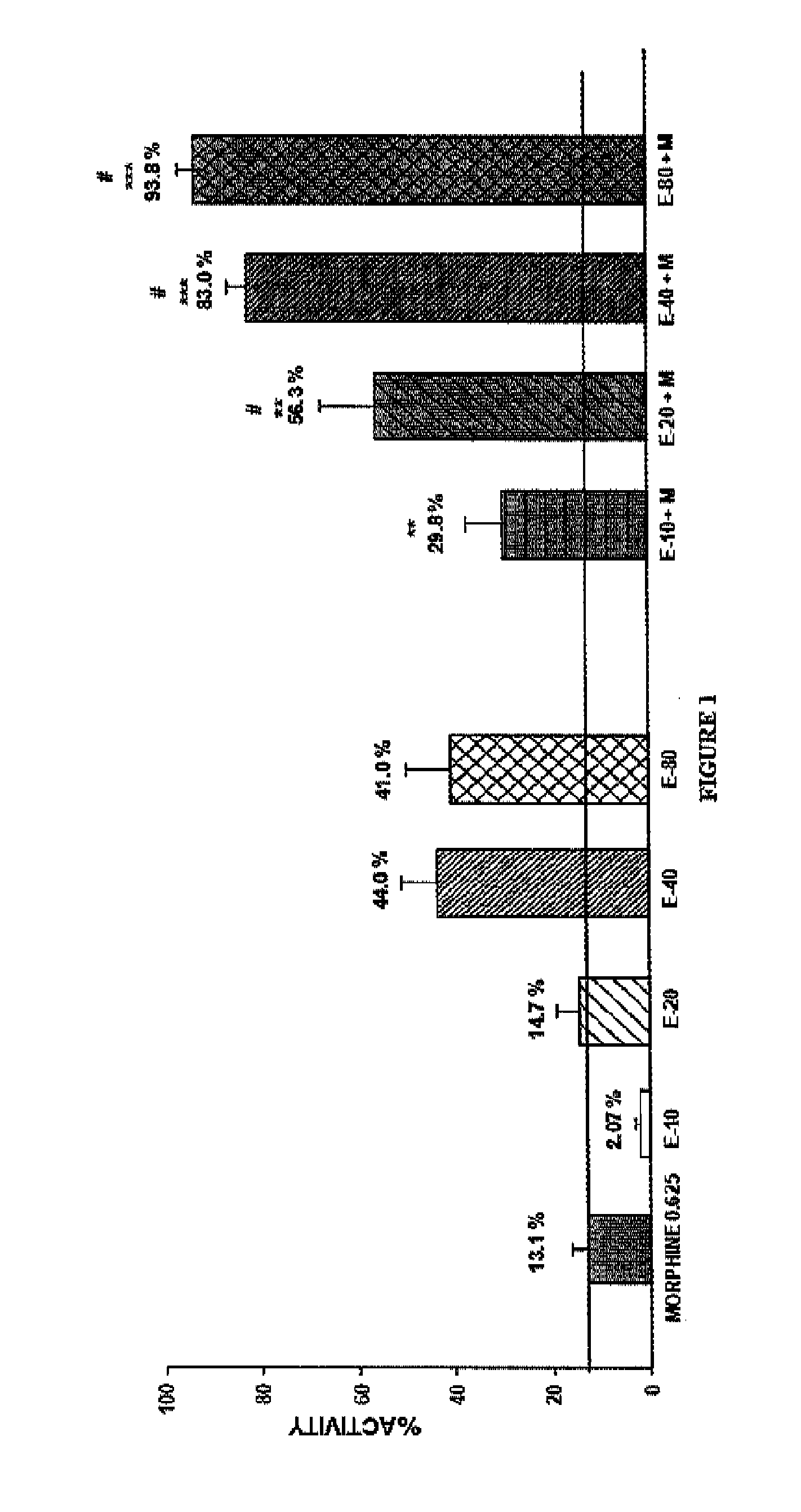
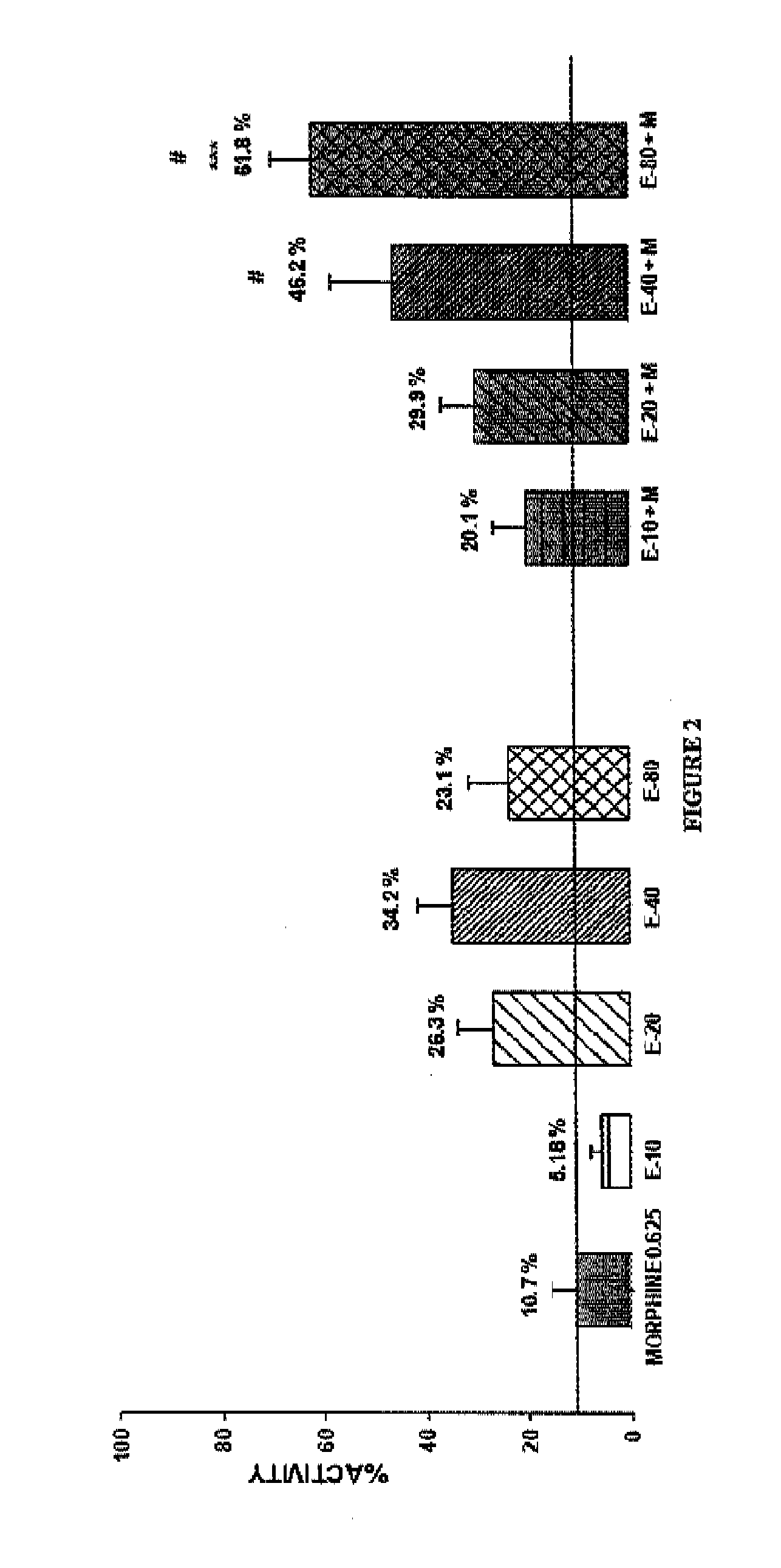
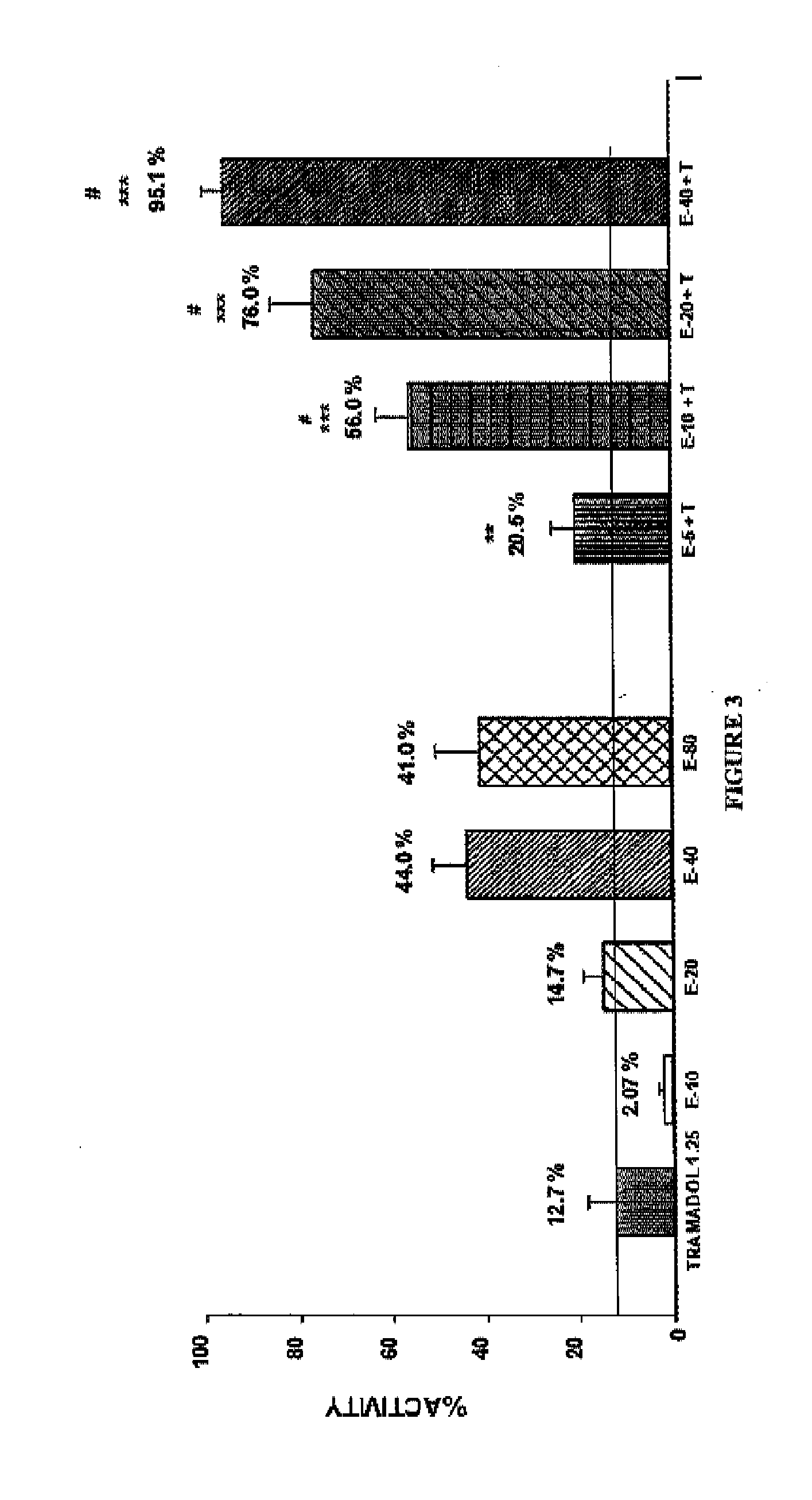
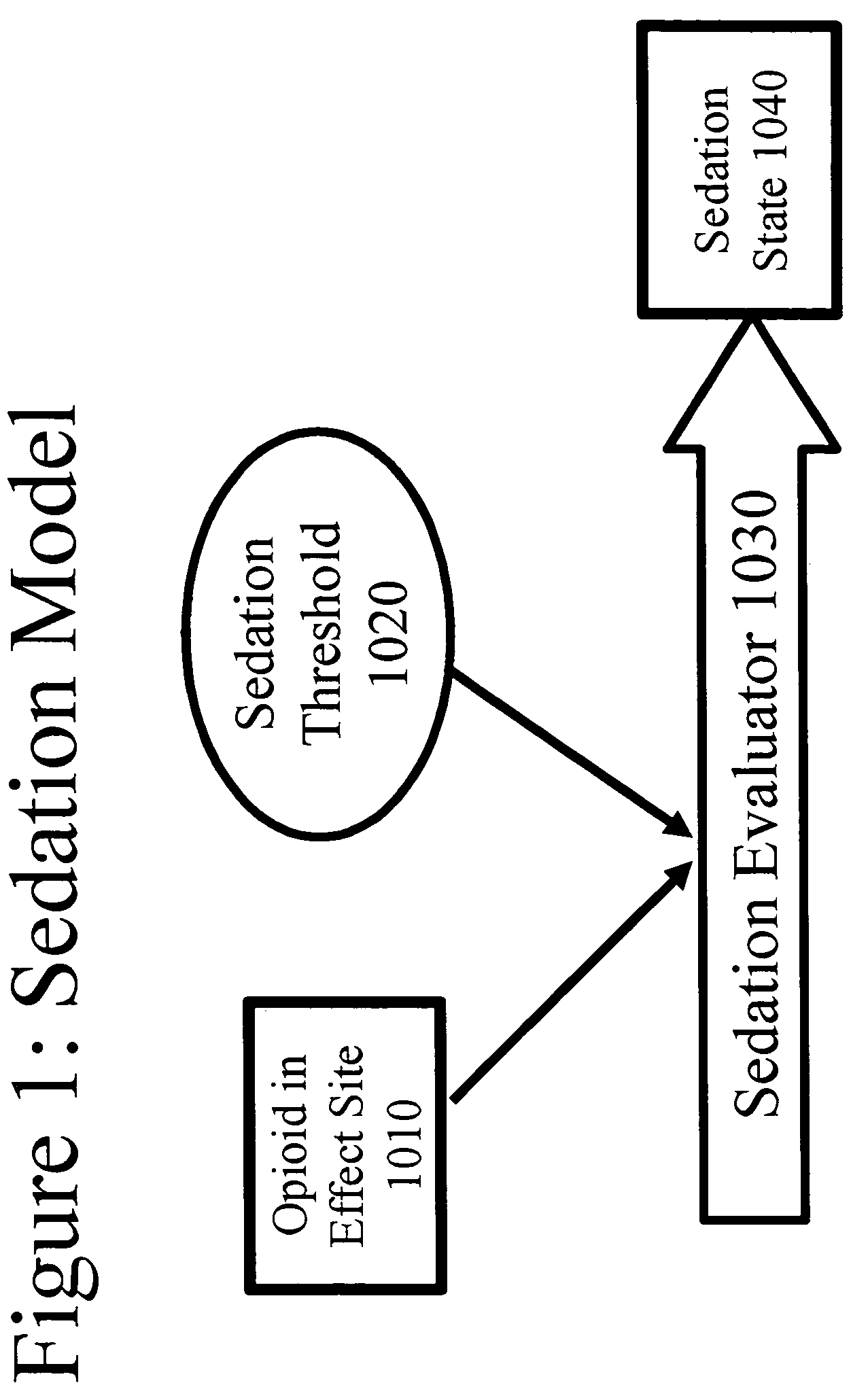
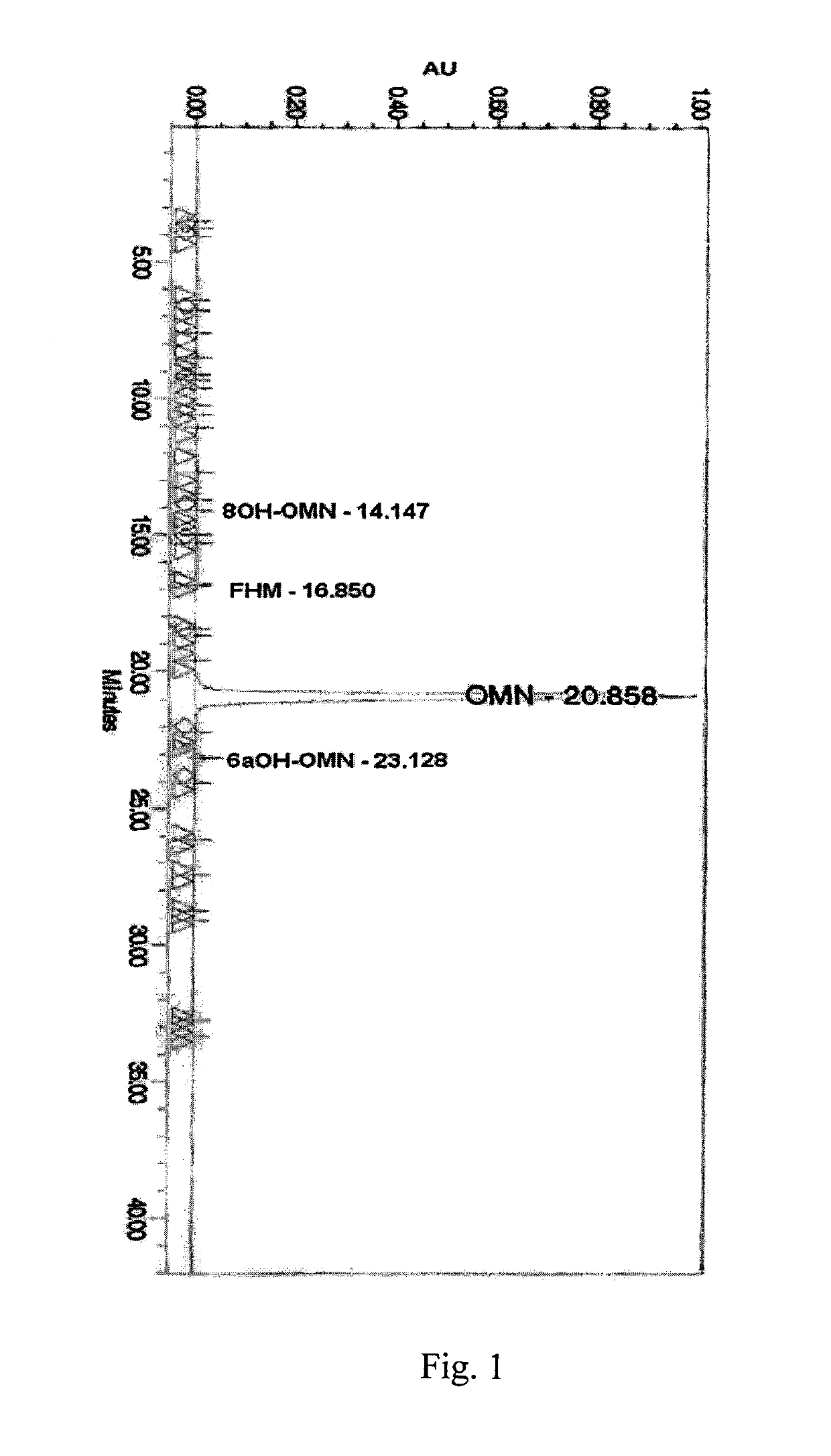
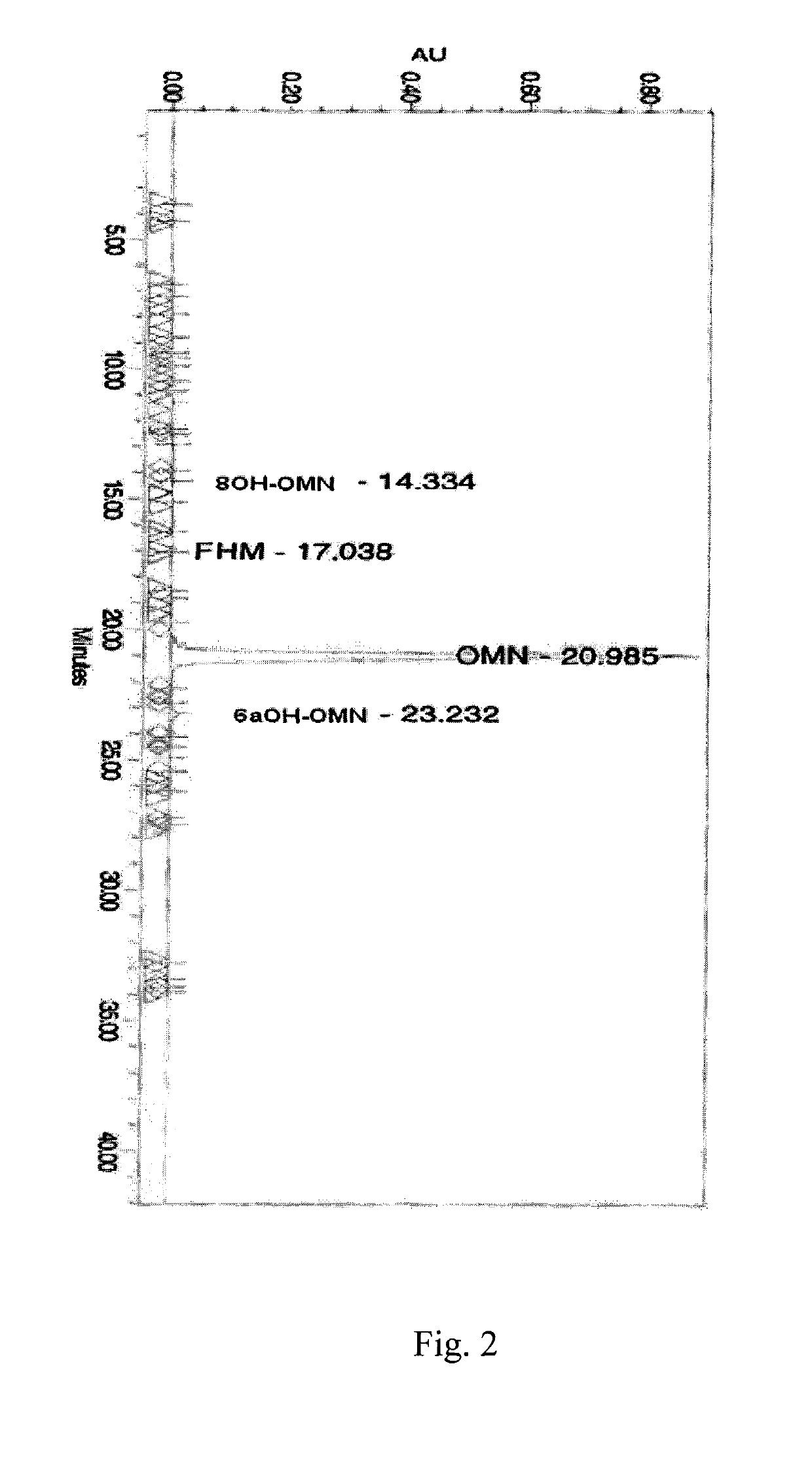
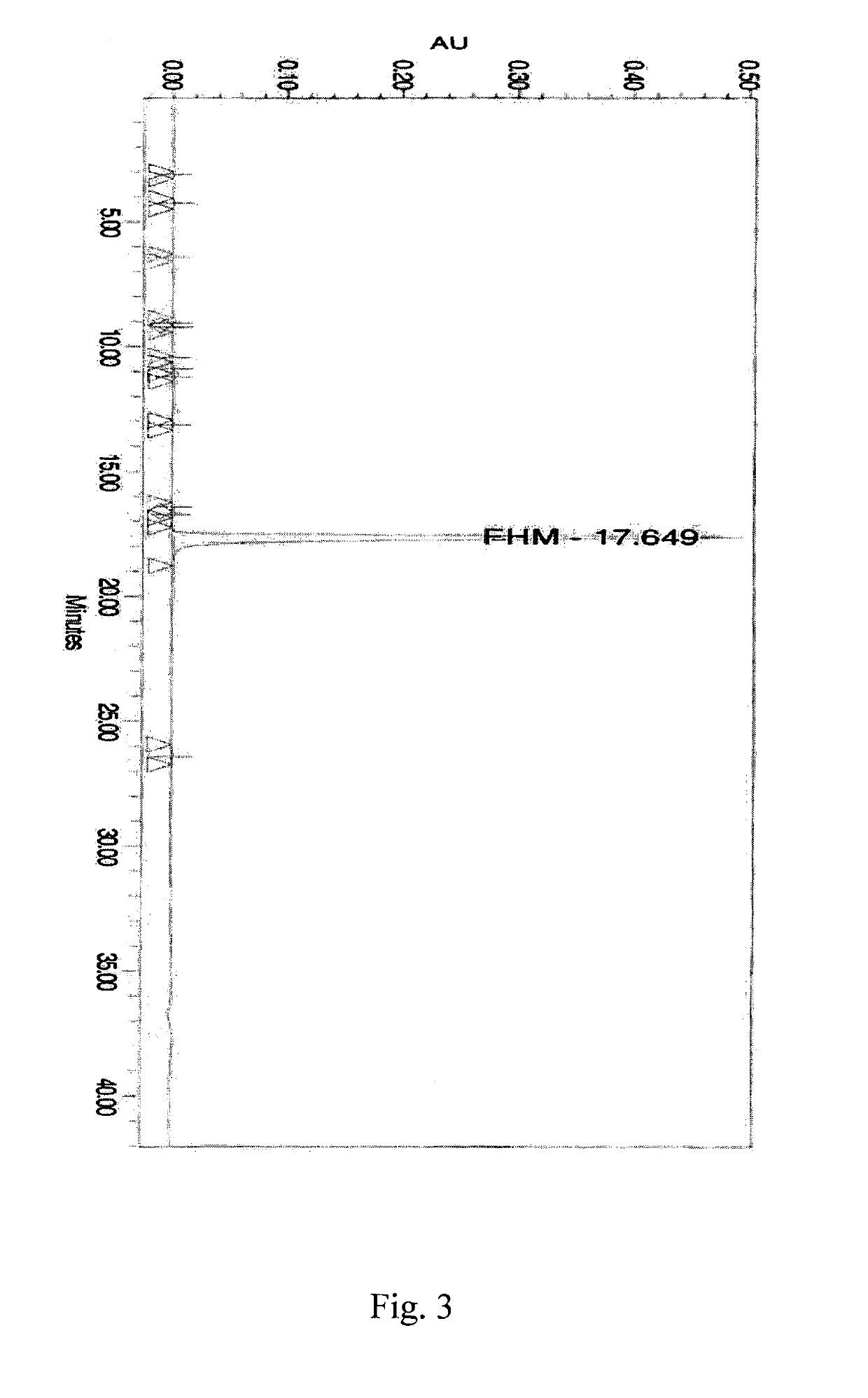
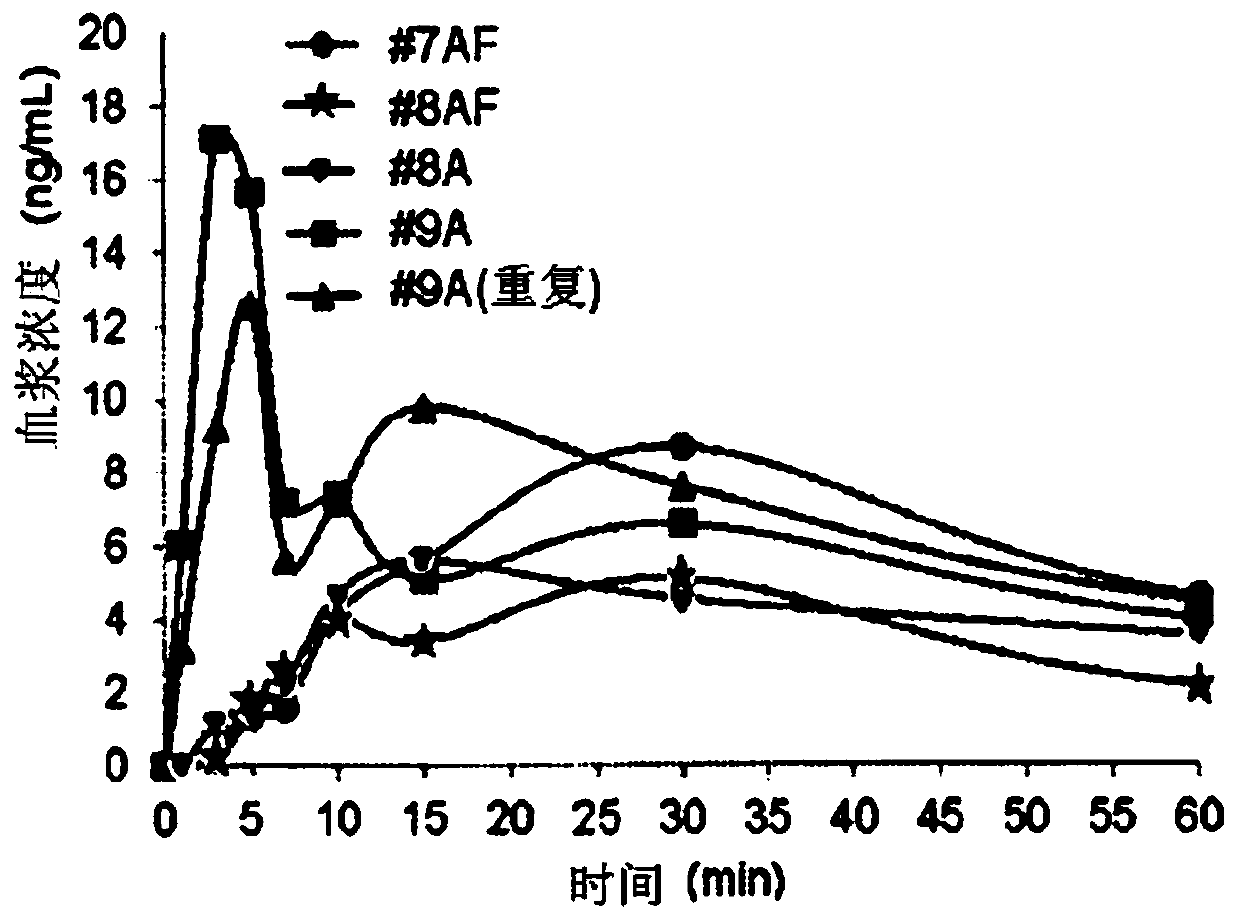
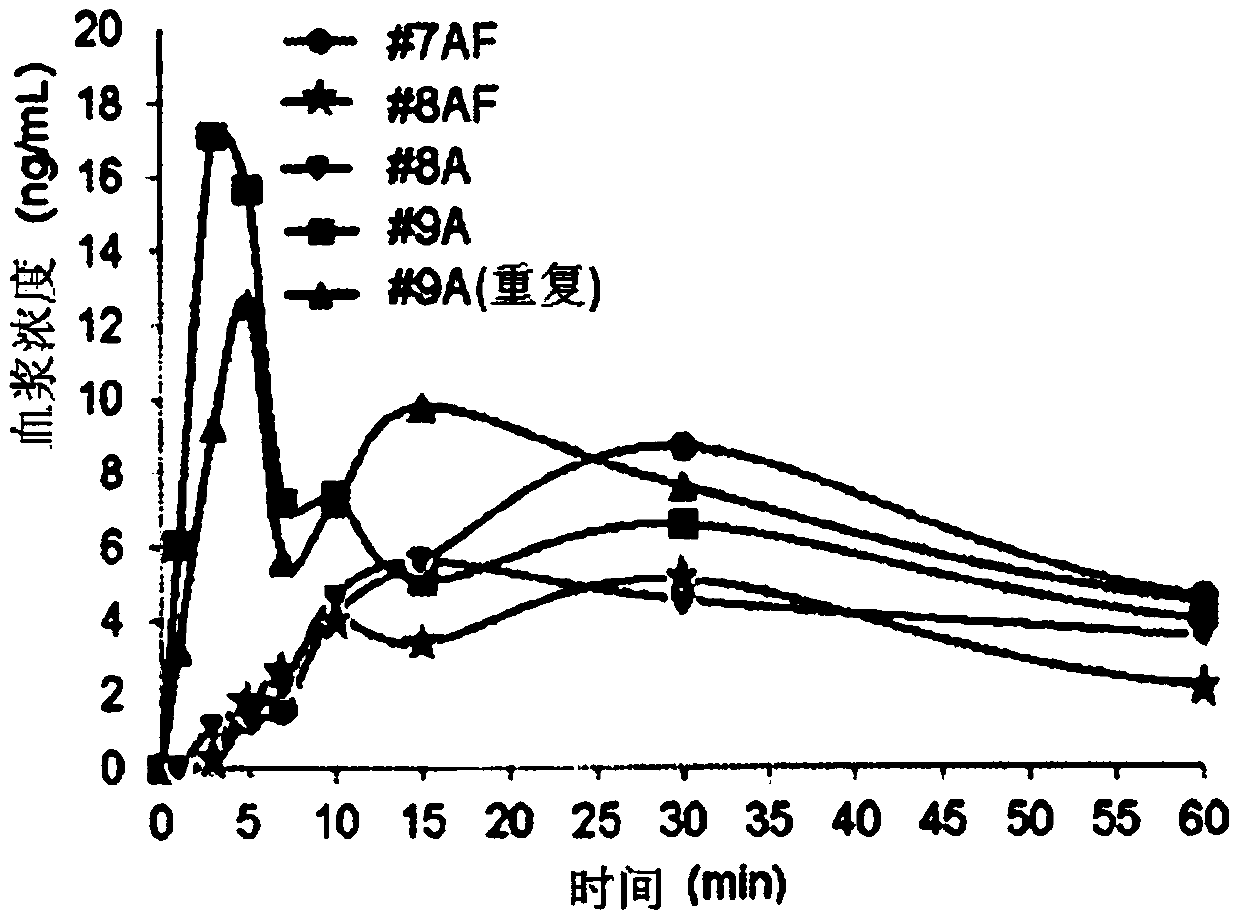
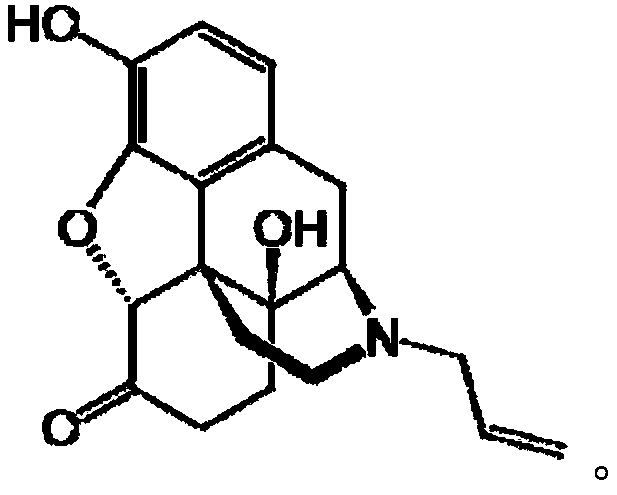
Patents
Literature
129 results about "Opioidergic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
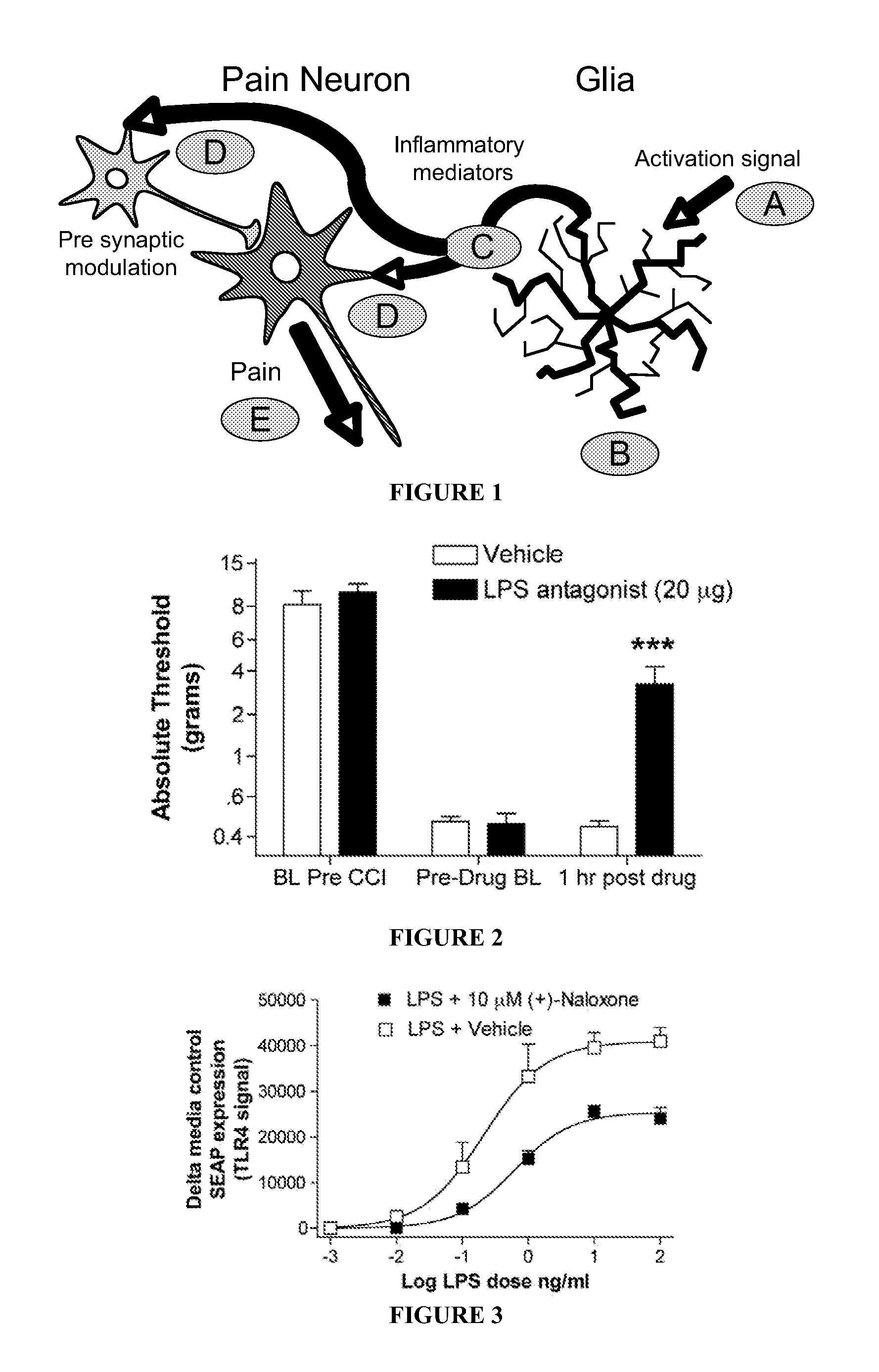
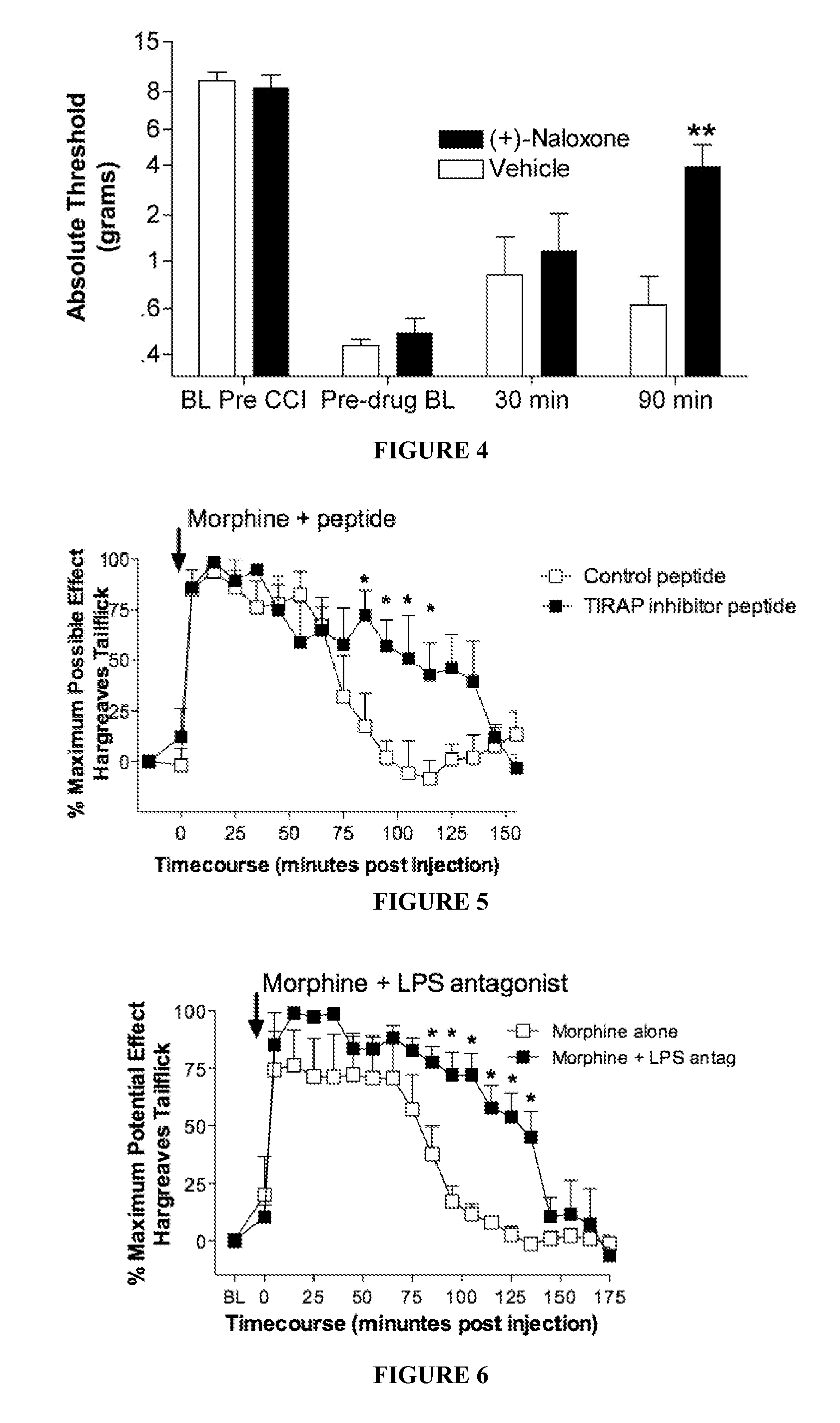
An opioidergic agent (or drug) is a chemical which functions to directly modulate the opioid neuropeptide systems (i.e., endorphin, enkephalin, dynorphin, nociceptin) in the body or brain. Examples include opioid analgesics such as morphine and opioid antagonists such as naloxone. Opioidergics also comprise allosteric modulators and enzyme affecting agents like enkephalinase inhibitors.
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
ActiveUS7399488B2Good treatment effectSmall dosePowder deliveryNervous disorderAdditive ingredientWater insoluble
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, a drug is modified to increase its lipophilicity. In preferred embodiments the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble, but enzymatically degradable by enzymes present in the human gastrointestinal tract. The abuse-deterrent composition retards the release of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
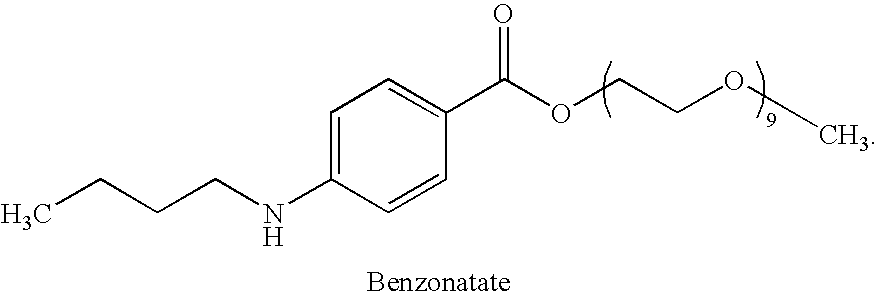
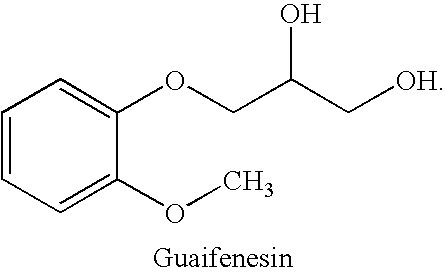
Combined administration of benzonatate and guaifenesin
Pharmaceutical compositions are provided containing a combination of benzonatate and guaifenesin, or pharmaceutically acceptable salts thereof. Methods of using compositions comprising benzonatate and guaifenesin, or pharmaceutically acceptable salts thereof, provide relief from cough, pulmonary congestion or both. The compositions and methods provide relief to opiate-sensitive individuals, in particular, as well as to infants and other pediatric patients.
Owner:VICTORY PHARMA
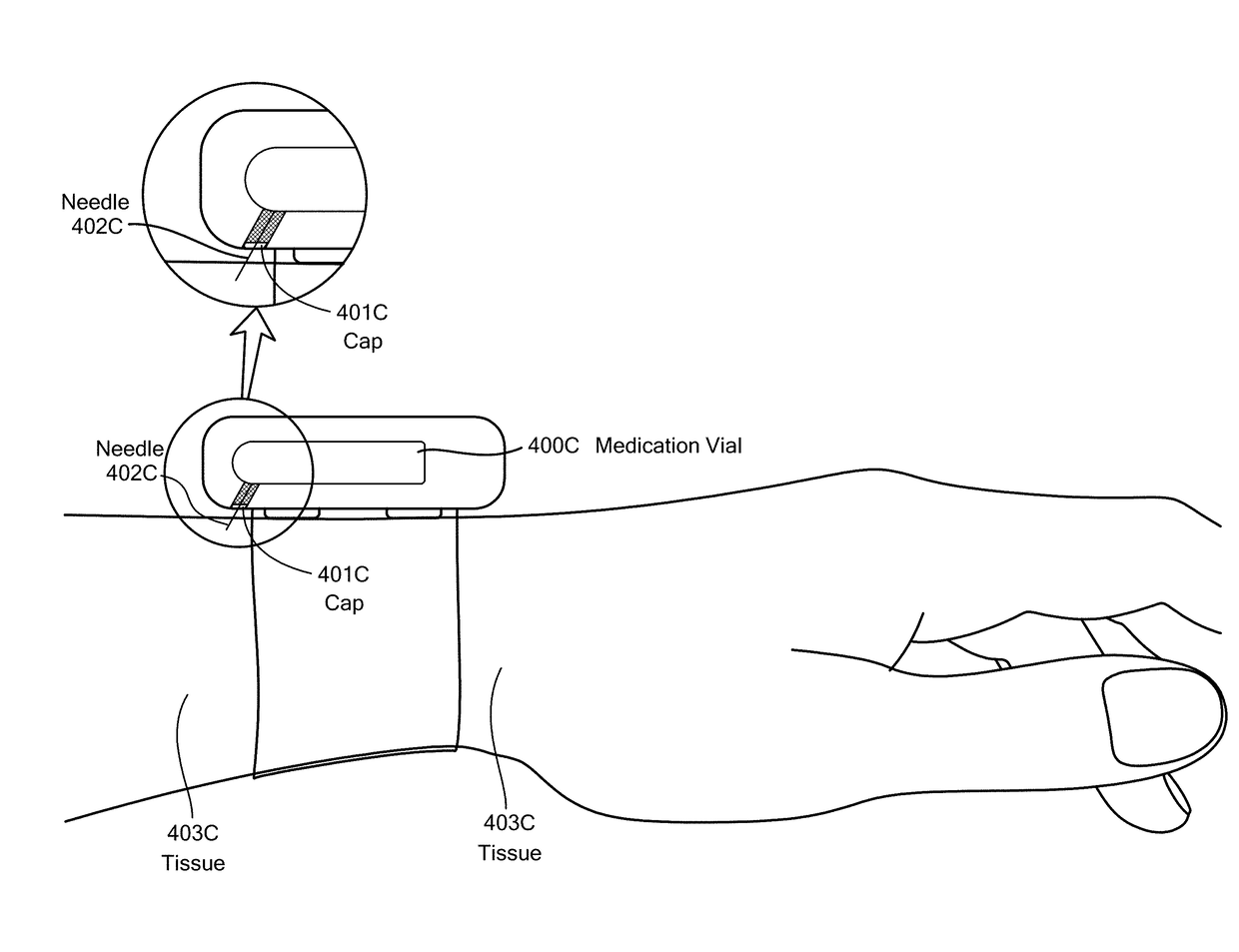
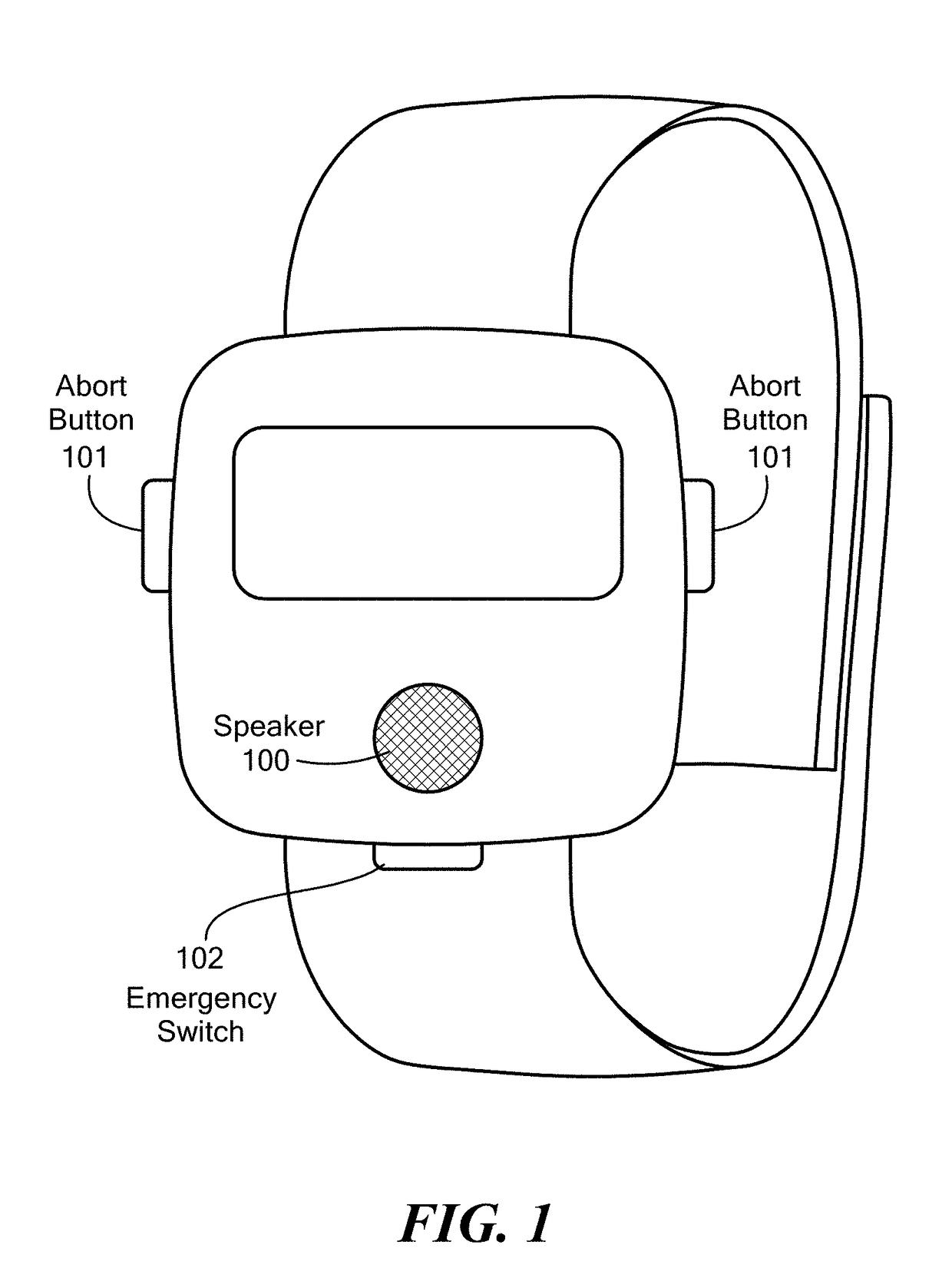
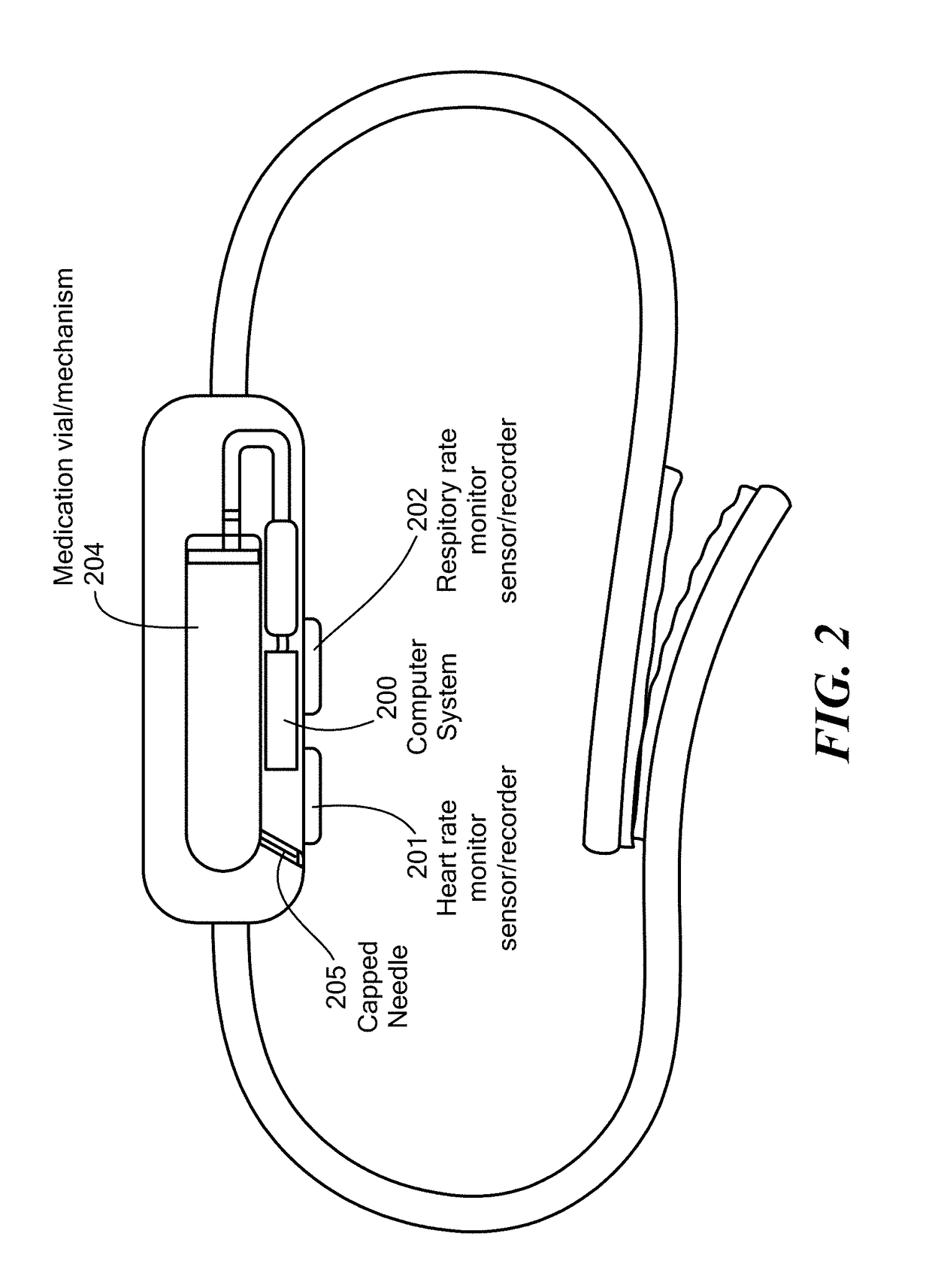
Method and Device for Automatic Identification of an Opioid Overdose and Injection of an Opioid Receptor Antagonist
ActiveUS20170172522A1Constant monitoringRespond effectivelyOrganic active ingredientsAutomatic syringesOpioidergicOpioid overdose
A method for detecting the need for providing assistance to an individual suspected of overdosing on an opiate is provided. The method includes using a wearable device for continuous or intermittent monitoring of one or more physiological parameters of the individual. If the level of one of the one or more physiological parameters exceeds a threshold level specific to that parameter, an alarm is triggered. If the alarm is not aborted, an alert is transmitted to one or more emergency contacts conveying that the individual has overdosed. The method optionally provides for injection of an opioid receptor antagonist into the individual to reverse the effects of the overdose. Also provided is a device for implementing these methods.
Owner:INSLER JOSEPH +1
Method of treating pain by administering 24 hour oral opioid formulations exhibiting rapid rate of initial rise of plasma drug level
InactiveUS20030035837A1Good analgesic effectQuick releaseBiocidePowder deliveryAbsorption Half-LifeOral medication
Patients are treated with 24-hour oral sustained release opioid formulations which, upon administration, provide an initially rapid opioid absorption such that the minimum effective analgesic concentration of the opioid is more quickly achieved. These sustained release opioid formulations include an effective amount of at least one retardant material to cause said opioid analgesic to be released at a such a rate as to provide an analgesic effect after oral administration to a human patient for at least about 24 hours, and are characterized by providing an absorption half-life from 1 to about 8 hours. A method of titrating a human patient utilizing these sustained release opioid formulations is also disclosed.
Owner:SACKLER RICHARD S +2
Methods and compositions for treating distress dysfunction and enhancing safety and efficacy of specific medications
InactiveUS20110159048A1Good treatment effectEliminate side effectsBiocideNervous disorderDiseaseNeurotransmitter systems
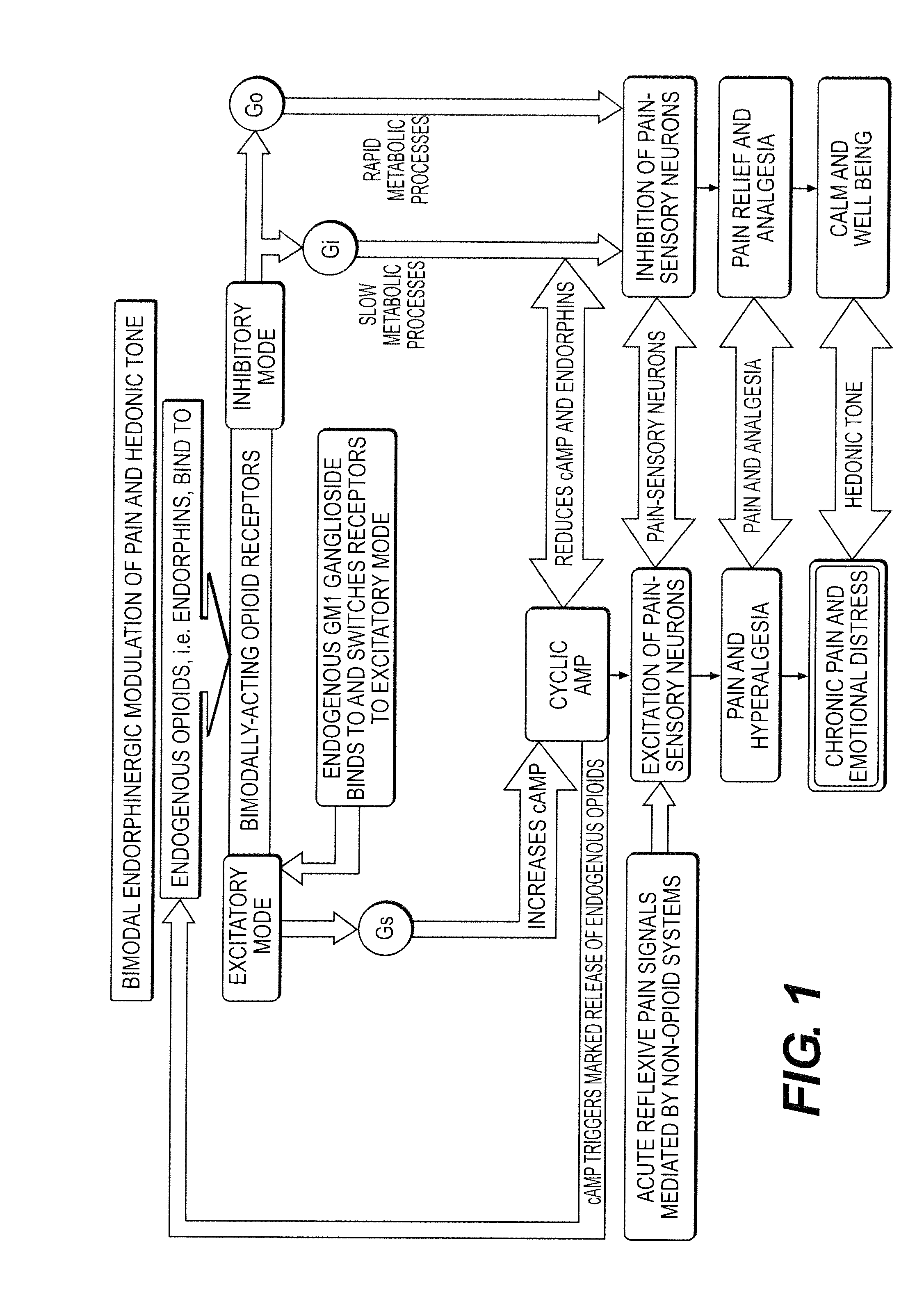
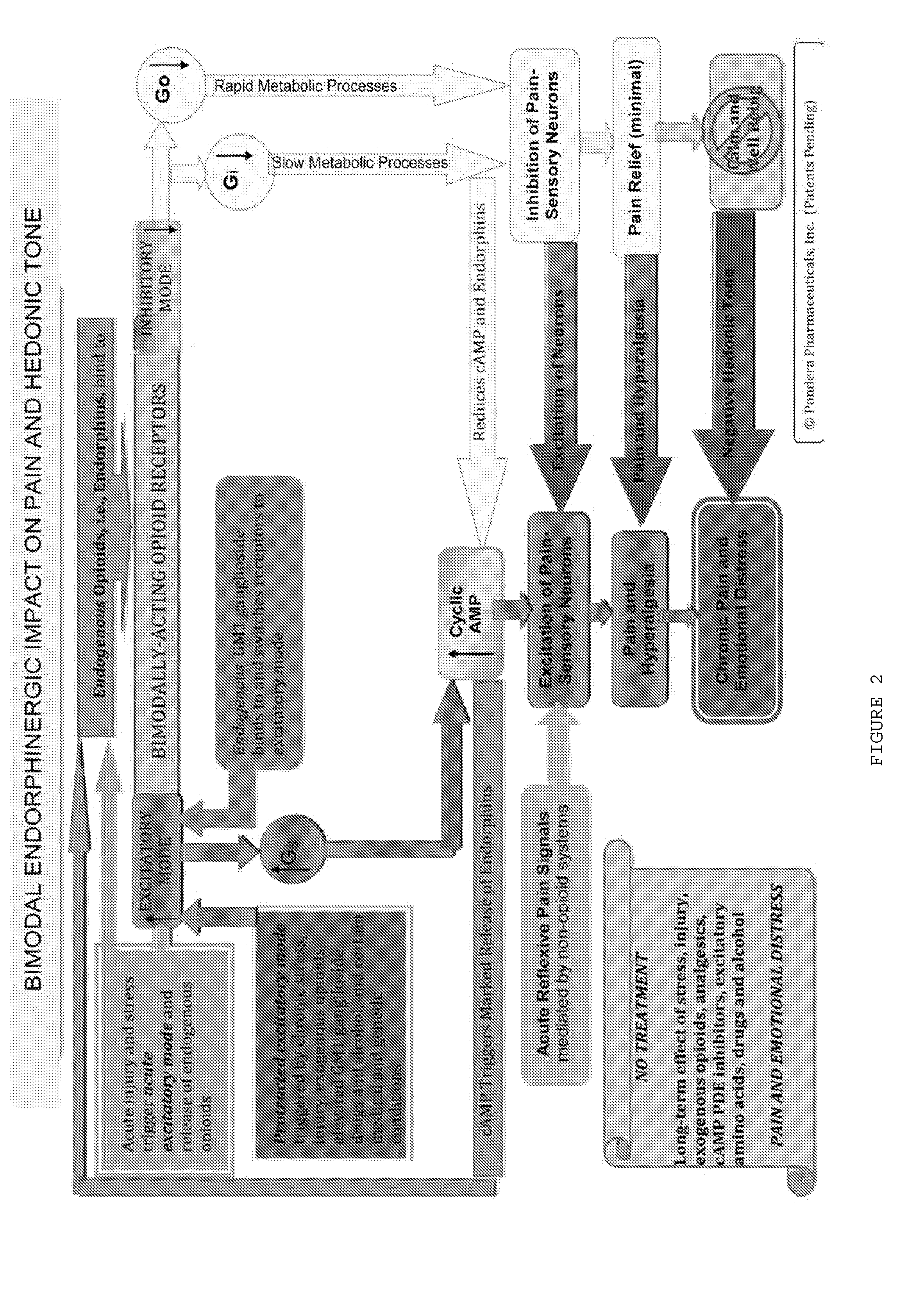
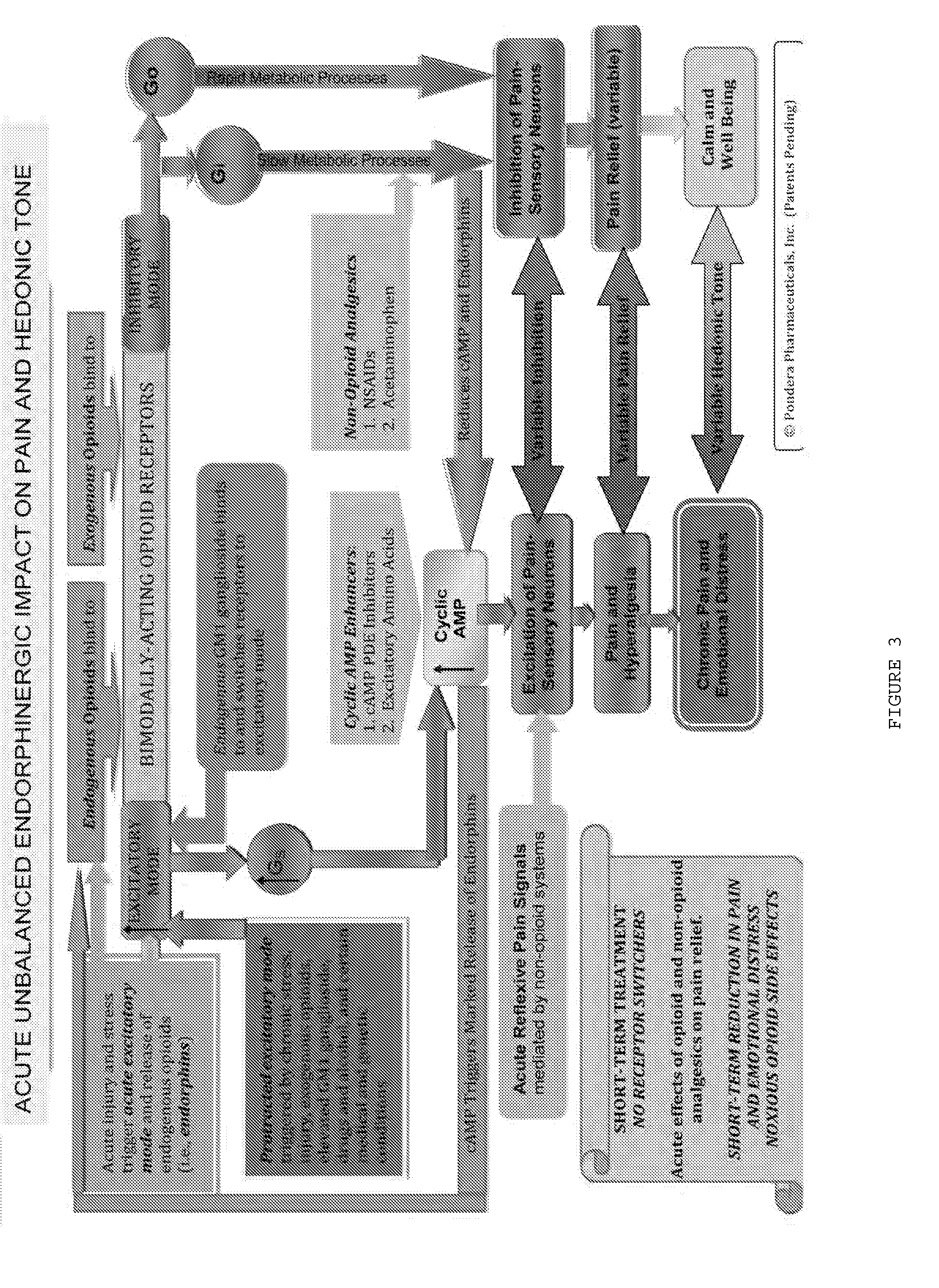
The present invention relates to methods and compositions for reducing Distress Dysfunction by restoring and maintaining homeostatic balance in the neurotransmitter systems underlying the Stress Response and the experience of distress and hedonic tone. Distress Dysfunction refers to the experience of dysfunctional emotional and physical distress that interferes with the individual's quality of life and functioning. A novel understanding of the bimodal opioid modulation of pain, and its impact, through serotonergic, dopaminergic, epinephrinergic, and norepinephrinergic processes, on hedonic tone, leads directly to new generation pharmaceutical formulations that are remarkably safe and effective for the treatment of a wide variety of Distress Dysfunctions, including anxiety, depression, anger, insomnia, mood disorders, eating disorders, sexual problems, pain, substance and behavioral addictions, gastrointestinal disorders, autistic spectrum disorders, attention-deficit and hyperactivity disorders, and other emotional and physical distress disorders. The foundation of this discovery is the power of Receptor Switchers, such as ultra-low-dose and very-low-dose opioid antagonists and GM1 ganglioside attenuators, in blocking acute and protracted excitatory opioid receptor signaling. Co-administration of Receptor Switchers with Endorphin Enhancers, such as specific cAMP PDE inhibitors and excitatory amino acids, is an excellent formulation for restoring healthy homeostatic balance to the endogenous opioid system, using the body's endorphins to reduce emotional and physical distress, and through synergistic and homeostatic processes, restoring positive hedonic tone. The addition of Synergistic Enhancers, such as amino acids, SSRI and SNRI agents, and non-opioid analgesics, as well as Exogenous Opioids, enhances and prolongs these therapeutic benefits. The novel principles discovered by this invention also teach a new generation of safe and effective formulations for the treatment of respiratory conditions, neuropathy, and nociceptive pain.
Owner:PONDERA BIOTECH
Large Substituent, Non-Phenolic Opioids
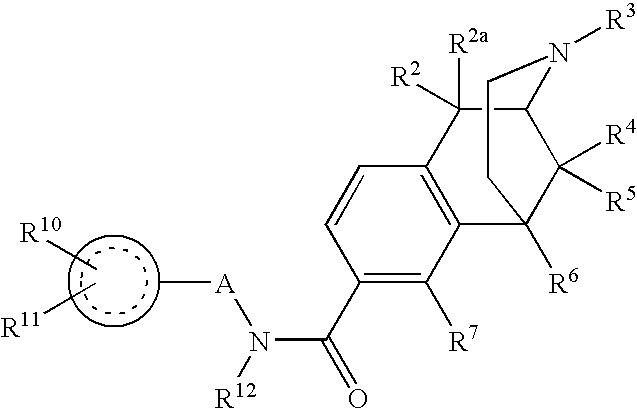
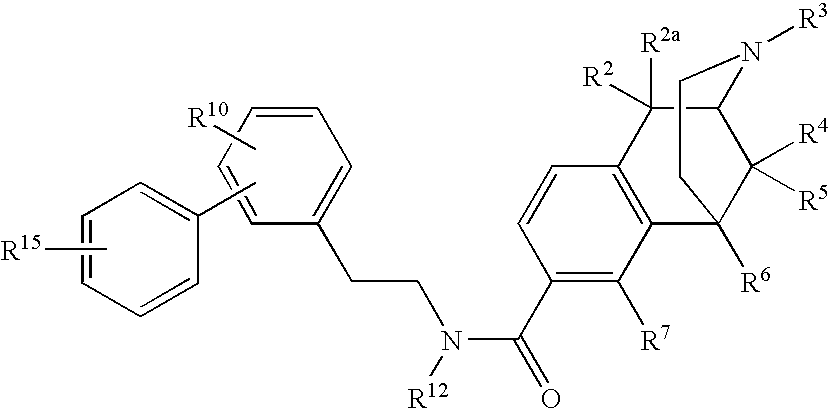
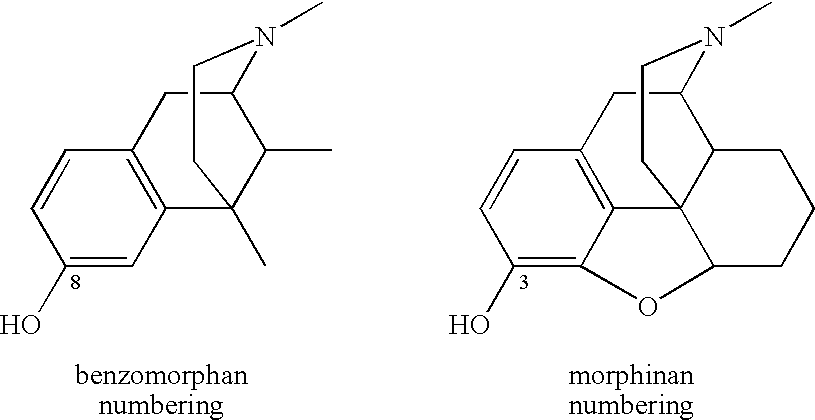
ActiveUS20070021457A1Excellent opioid bindingGood metabolic stabilityBiocideNervous disorderBenzazocineAnalgesic agents
8-Substituted-2,6-methano-3-benzazocines of general structure are useful as analgesics, anti-diarrheal agents, anticonvulsants, antitussives and anti-addiction medications. One embodiment is the subgenus of biphenylethyl compounds:
Owner:RENESSELAER POLYTECHNIC INST
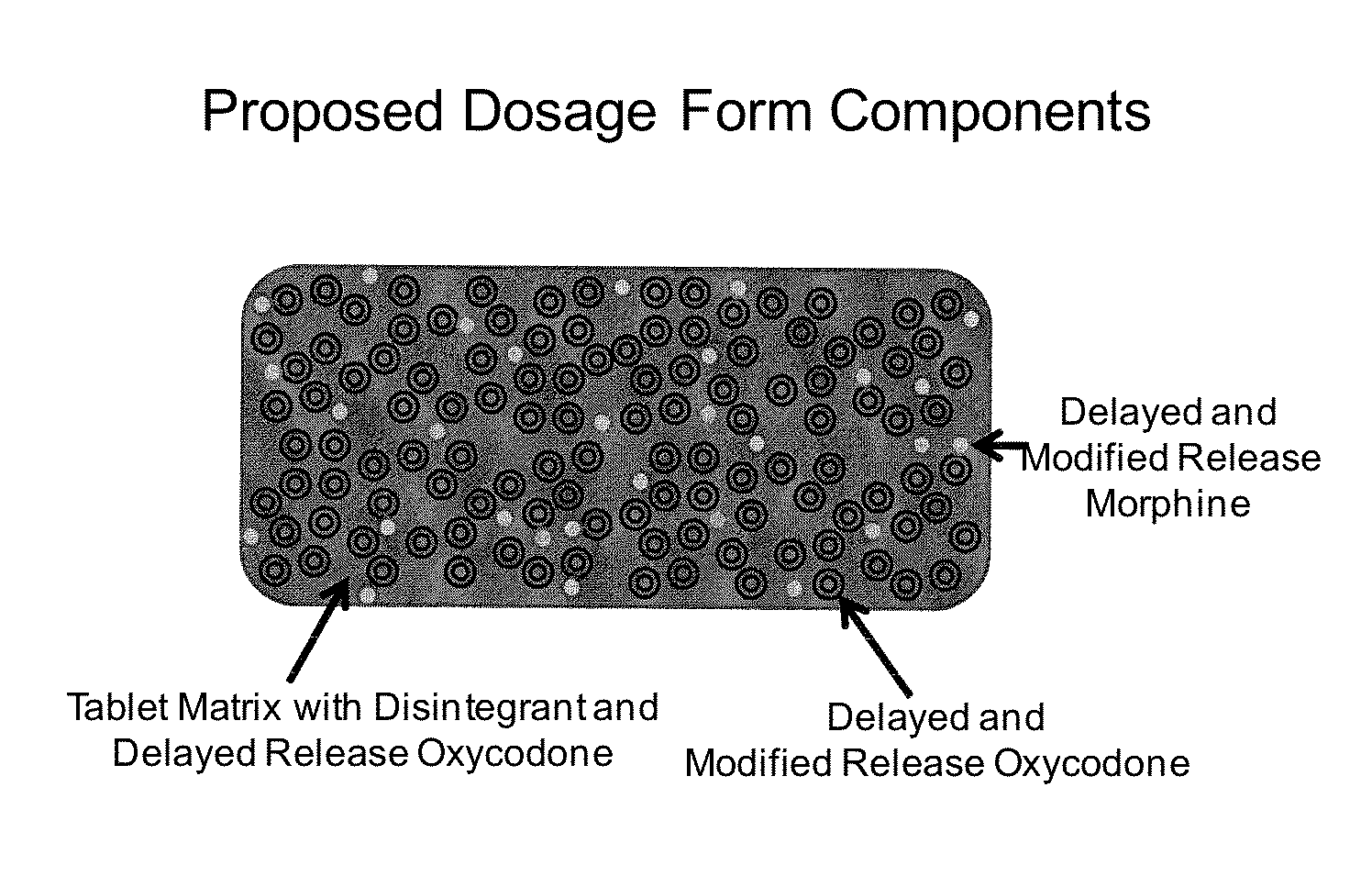
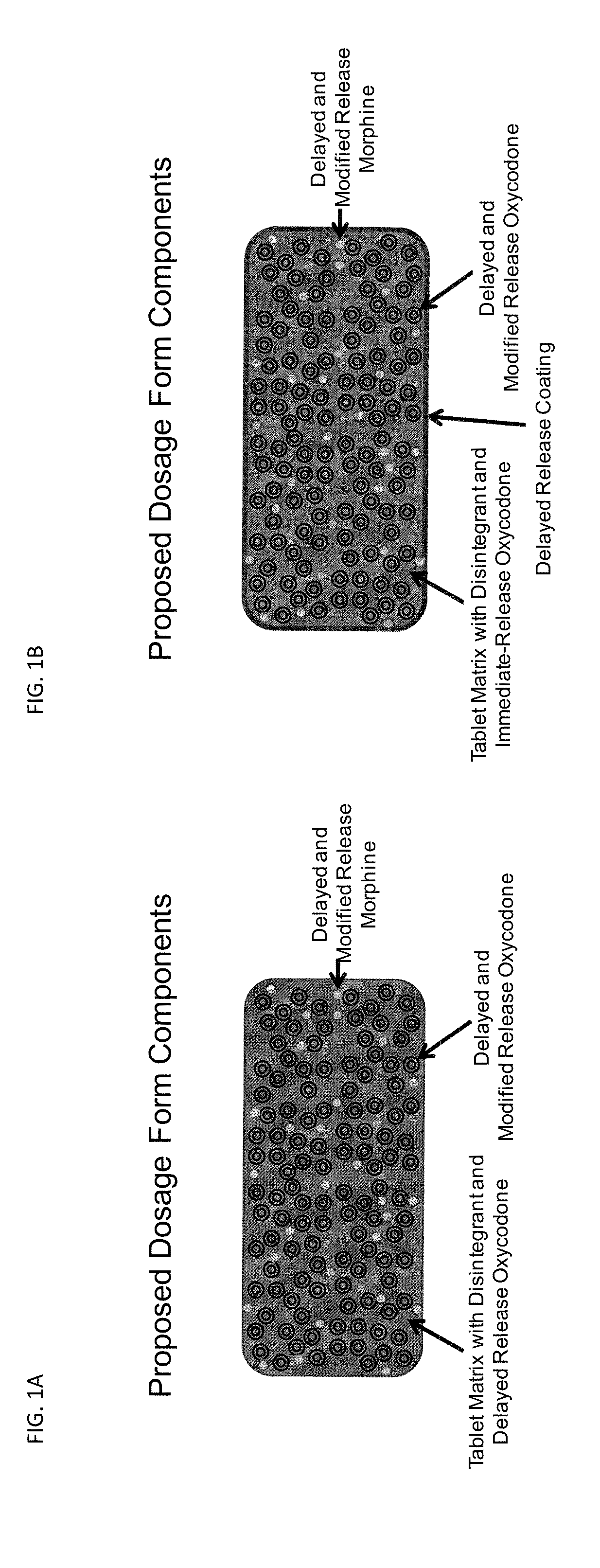
Controlled Release Formulations of Opioids
InactiveUS20130022646A1BiocideNervous disorderPharmaceutical formulationControlled-Release Formulations
Pharmaceutical formulations containing opioid components that each has a release profile. The components may provide immediate or controlled release of the opioid. The invention is also directed to methods of controlling release of one or more opioid compounds and methods of treating pain.
Owner:QRXPHARMA
Abuse-deterrent pharmaceutical compositions of opioids and other drugs
InactiveUS20080199530A1Reduce the possibilityImprove lipophilicityPowder deliveryNervous disorderAdditive ingredientWater insoluble
Owner:COLLEGIUM PHARMA INC
(+)-opioids and methods of use
The invention provides 4,5-epoxymorphinan or a derivative thereof, a morphinan or a derivative thereof, or a pharmaceutical salt or a prodrug thereof. The present invention also provides compositions comprising the same, and methods for using the same. In particular, the invention relates to TLR antagonistic opioids and methods for using the same.
Owner:UNIV OF COLORADO THE REGENTS OF
Pharmaceutical composition for nasal delivery
ActiveUS10653690B1Improve bioavailabilityFast absorptionOrganic active ingredientsNervous disorderOpioid antagonistOpioidergic
There is provided a solid pharmaceutical composition formulation for nasal delivery of an opioid antagonist. The pharmaceutical composition includes a pharmacologically-effective amount of an opioid antagonist and a pharmaceutically-acceptable carrier. The pharmaceutical composition is preferably in the form of a powder produced by spray-drying, which is subsequently loaded into single use nasal applicators. Preferred pharmaceutically-acceptable carriers include disaccharides (e.g. lactose or trehalose) and dextrins (e.g. cyclodextrins or maltodextrins), preferably spray-dried together in combination. The pharmaceutical composition may further comprise an alkyl saccharide, preferably a sucrose ester, such as sucrose monolaurate. The compositions and applicators may be employed in the treatment of opioid overdose in subjects.
Owner:OREXO AB
Prodrugs of opioids and uses thereof
InactiveUS20110190267A1Improve oral bioavailabilityReduced bioavailabilityBiocideNervous disorderSide effectBioavailability
The present invention concerns prodrugs of opioid analgesics and pharmaceutical compositions containing such prodrugs. Methods for providing more consistent pain relief by increasing the bioavailability of the opioid analgesic with the aforementioned prodrugs are provided. The invention also provides for decreasing the adverse GI side effects of opioid analgesics.
Owner:SHIRE PHARMA INC
Transdermal drug delivery device for delivering opioids
Systems and methods are provided herein for transdermal delivery of an opioid. The opioids can be delivered in a decreasing dose (dose-tapering) manner to minimize withdrawal symptoms and abuse. The systems are also designed to limit a person besides the patient from receiving the drug with the transdermal delivery device. The systems can also include abuse-deterrence features that limit the ability of a patient or unauthorized user from tampering with the device to receive the active ingredient. Novel transdermal drug delivery formulations are also provided that include an opioid agonist or partial agonist and opioid antagonist which prevent the abuse of formulation.
Owner:MORNINGSIDE VENTURE INVESTMENTS
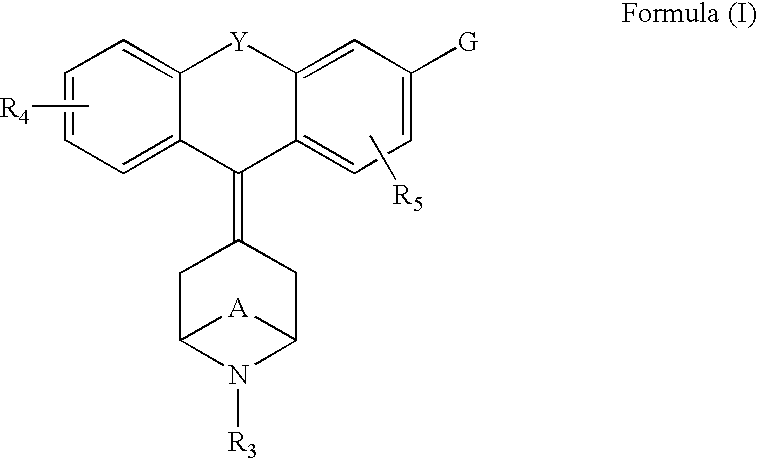
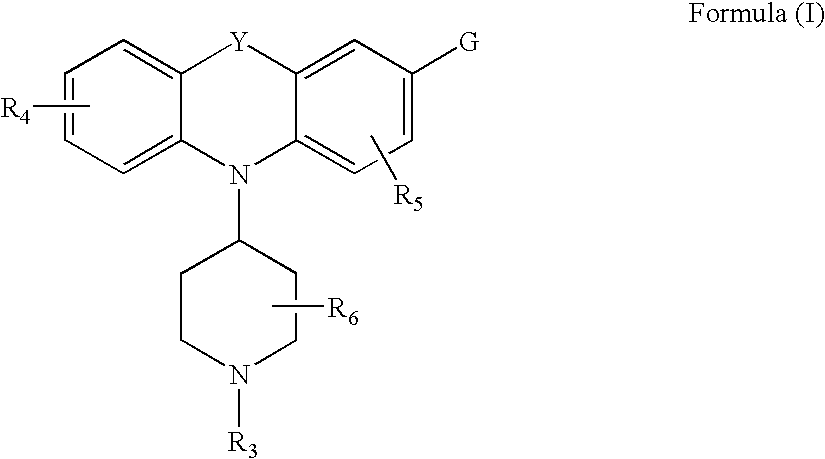
Tricyclic-bridged piperidinylidene derivatives as delta-opioid modulators
Owner:JANSSEN PHARMA NV
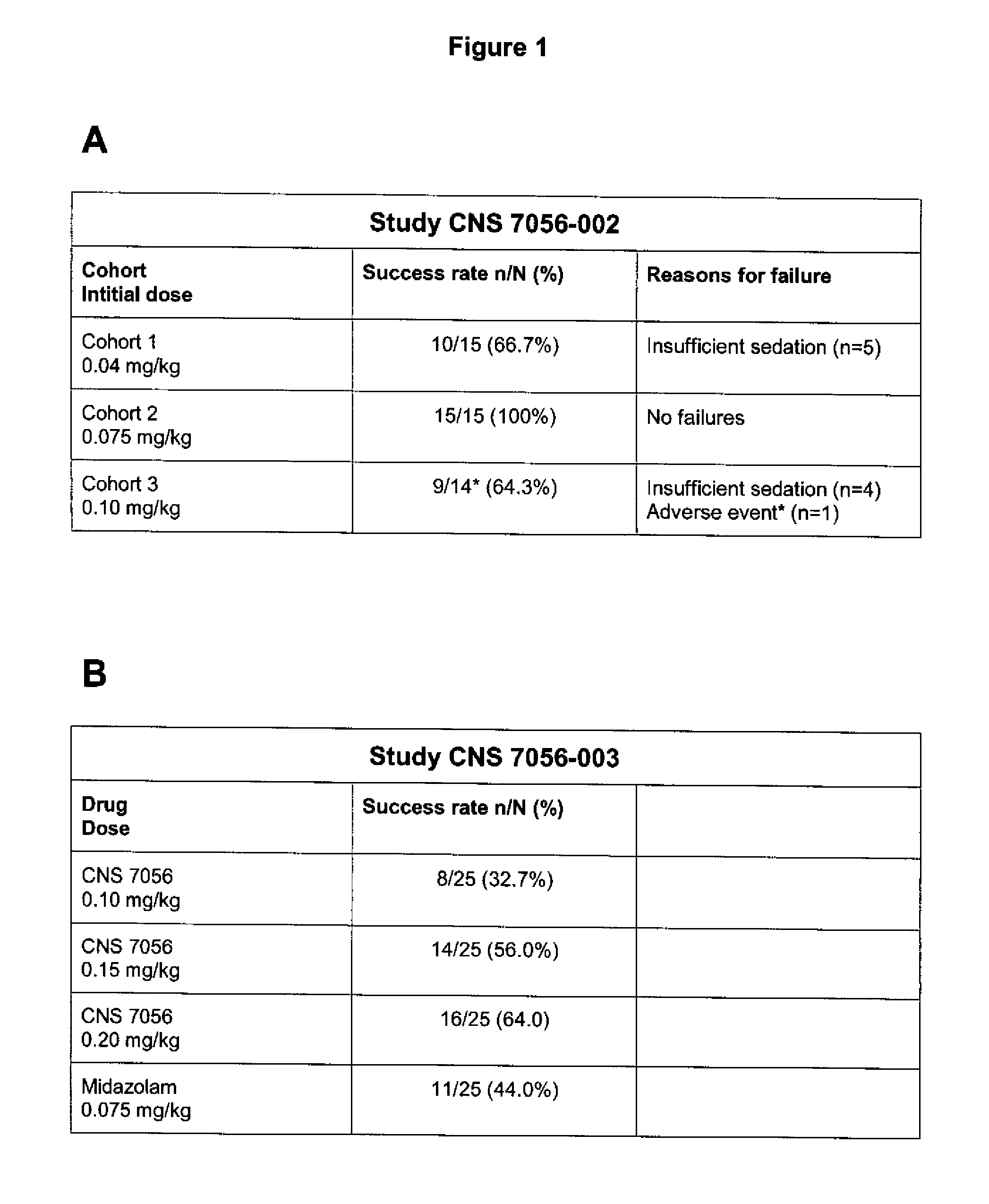
Dosing regimen for sedation with CNS 7056 (Remimazolam)
ActiveUS9561236B2Enhance the imageMinimizing rateOrganic active ingredientsNervous disorderDosing regimenRegimen
Owner:PAION UK
Opioid Combination Wafer
InactiveUS20090291123A1Improve complianceEfficient use ofNervous disorderAntipyreticPain therapyHydrophilic polymers
Sheet-like dosage forms for pain therapy, based on hydrophilic polymers, which rapidly dissolve or disintegrate in an aqueous environment and which release active agent combinations when placed into a body orifice or body cavity, and which are preferably orally administrable, with the dosage form containing an active agent combination consisting of an opioid and a second substance The second active agent is a non-steroidal anti-rheumatic (NSAR) or an antidepressant.
Owner:LTS LOHMANN THERAPIE-SYST AG
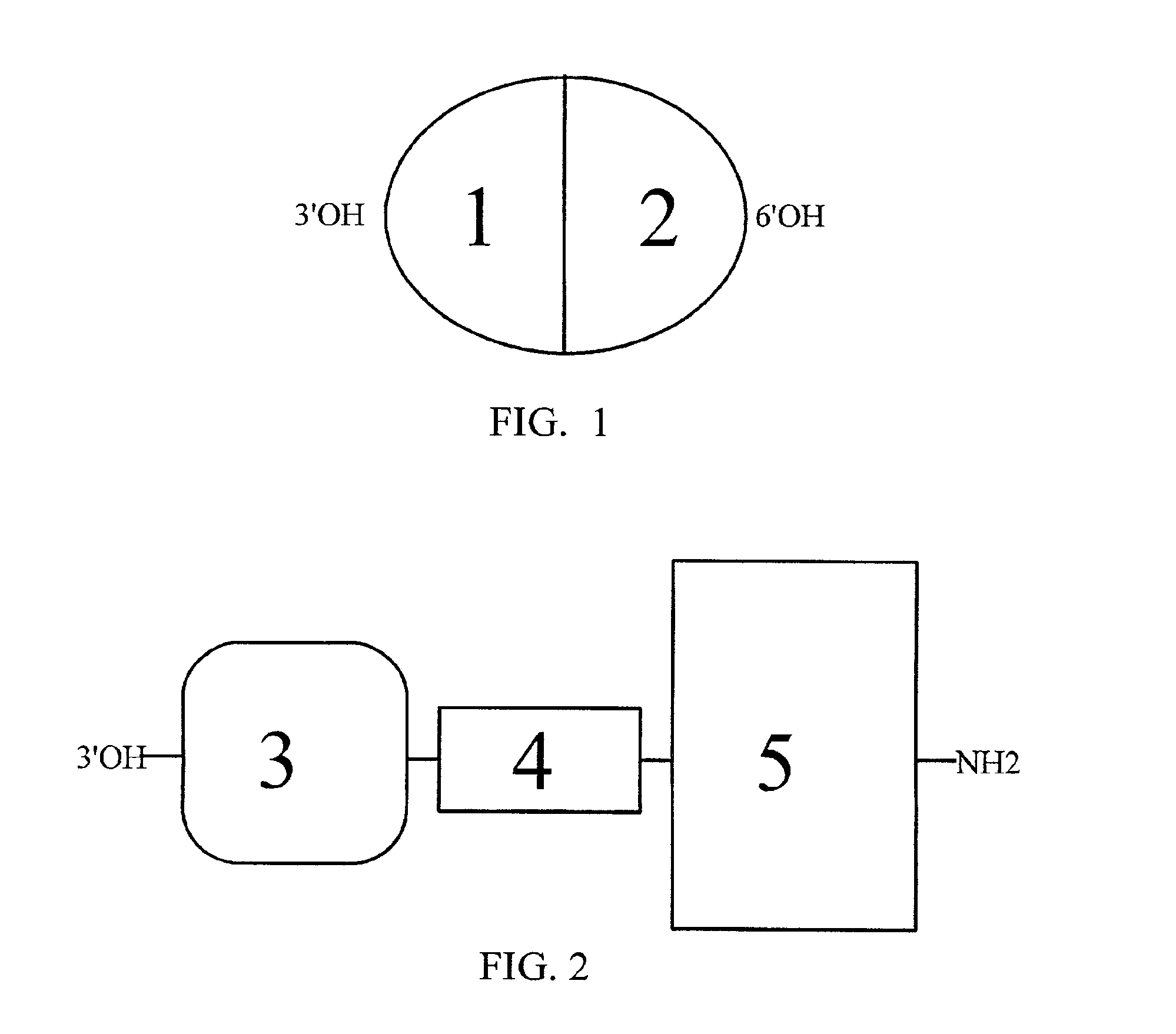
Chimeric hybrid analgesics
InactiveUS6881829B2Little and no developmentUndesirable side-effectNervous disorderAntipyreticTolerabilityWhole body
The present invention provides composition of matter for and methods of treating pain and drug abuse using novel chimeric hybrid molecules containing an opioid moiety of chemically modified morphine (3) that binds to and activates the human mu (μ) opioid receptor, with the opioid moiety linked through a novel linker-hinge (4) to a substance P peptide fragment moiety (5) that binds to and activates the human substance P receptor. The hybrid alkaloid / peptide chimeric molecules produce clinically efficacious opioid analgesia with little or no development of opioid tolerance or formation of opioid dependence. The hybrid alkaloid / peptide analgesics may be administered intrathecally, systemically or orally.
Owner:CHIMERACOM LLC
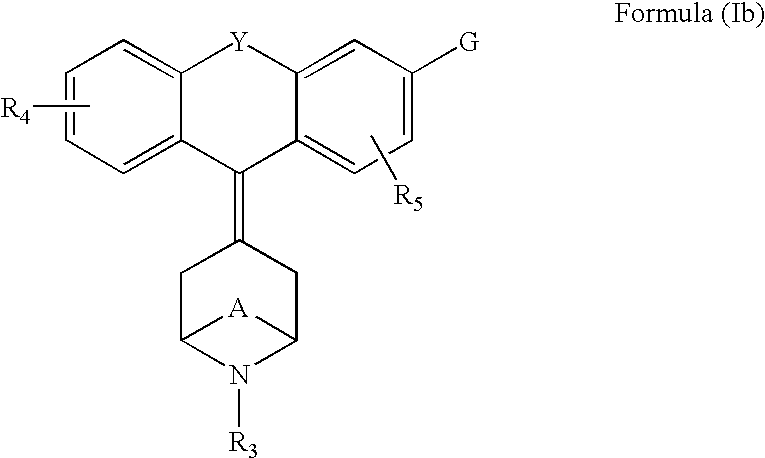
Piperdinyl-phenoxazine and phenothiazine derivatives as delta-opioid modulators
Owner:JANSSEN PHARMA NV
Biologically active cannabidiol analogs
Biologically active cannabidiol analogs comprising a compound of the formulawherein one of R1 or R2 or both is / are the residue of a moiety formed by the reaction of an amino group of the amino acid ester of R1 or R2 or both with a dicarboxylic acid or a dicarboxylic acid derivative and the other R1 or R2 (in the case of the mono) is the residue of a dicarboxylic acid or dicarboxylic acid derivative or Hydrogen (H), (i.e. underivatized), and salts thereof. These CBD analogs are be useful in pain management in oncology and other clinical settings in which neuropathy is presented. Furthermore, these CBD-analogs are useful in blocking the addictive properties of opiates.
Owner:UNIVERSITY OF MISSISSIPPI
Oil-phase preparation for opiates medicaments and preparation method thereof
InactiveCN102133406AShort duration of pain reliefSolving a world-wide problem prone to addictionNervous disorderOil/fats/waxes non-active ingredientsSide effectOpioidergic
The invention relates to an oil-phase preparation for opiates medicaments and a preparation method thereof. The preparation is oral administration oil prepared by fully dissolving the opiates medicaments into medical or edible oil. By changing the formulations and the administration mode of the opiates medicaments, the dependency and tolerance of the opiates medicaments are controlled effectively. The oil-phase preparation is used for easing pain, and can reduce the using dose of the opiates medicaments, prolong the analgesis time, improve the analgesis effect and reduce or eliminate the tolerance, dependency and the toxic and side effect of the opiates medicaments.
Owner:泰州市康特生物工程有限公司
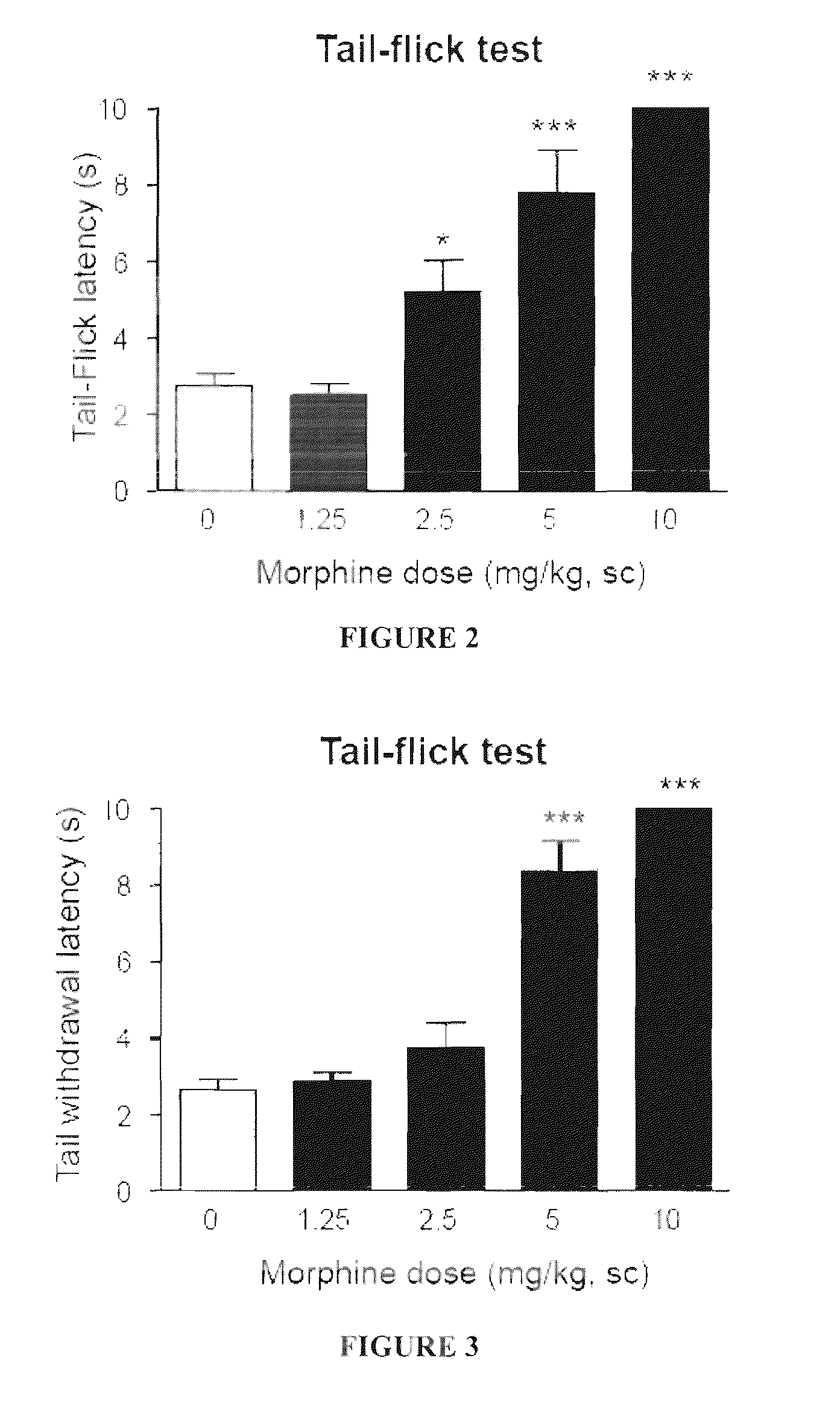
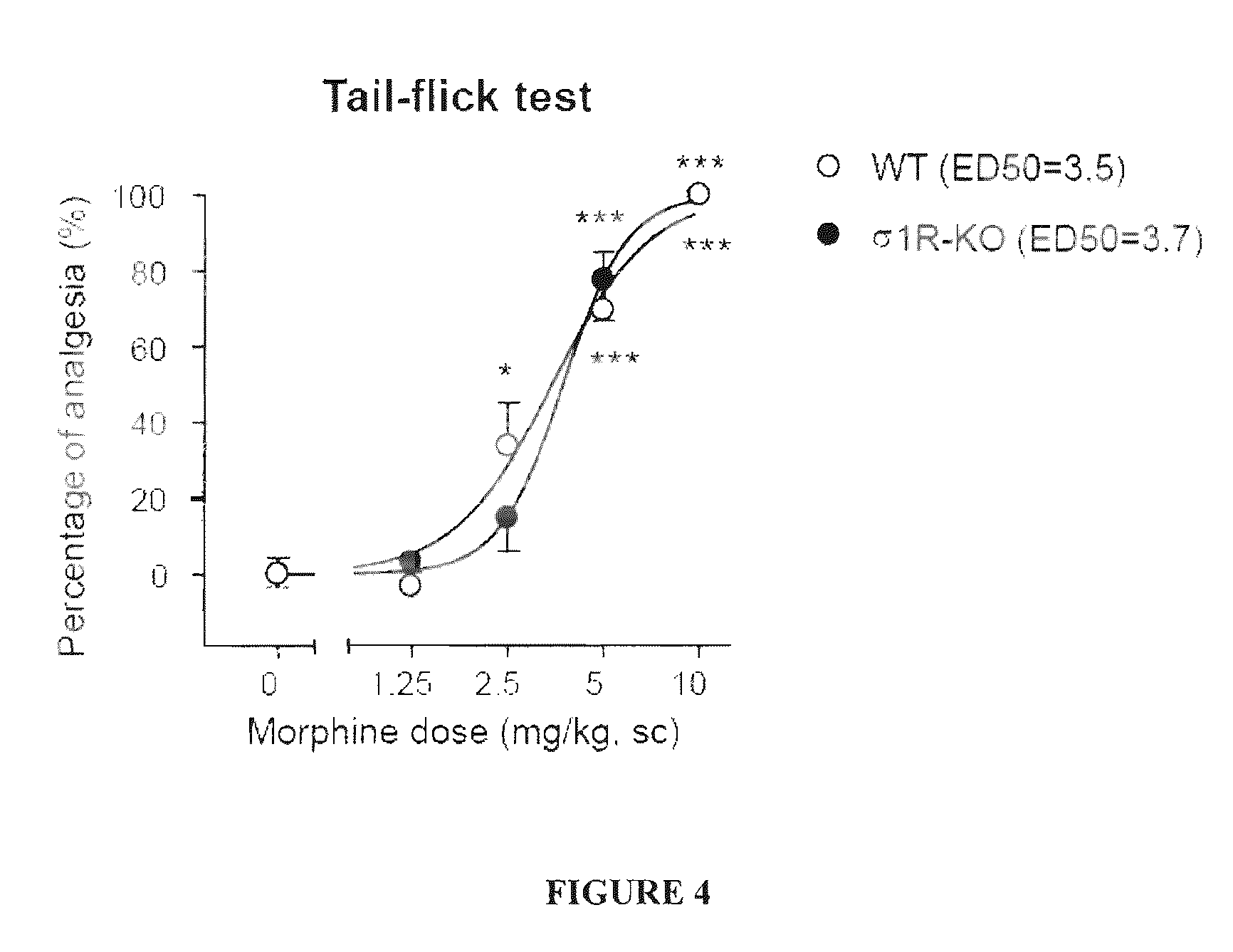
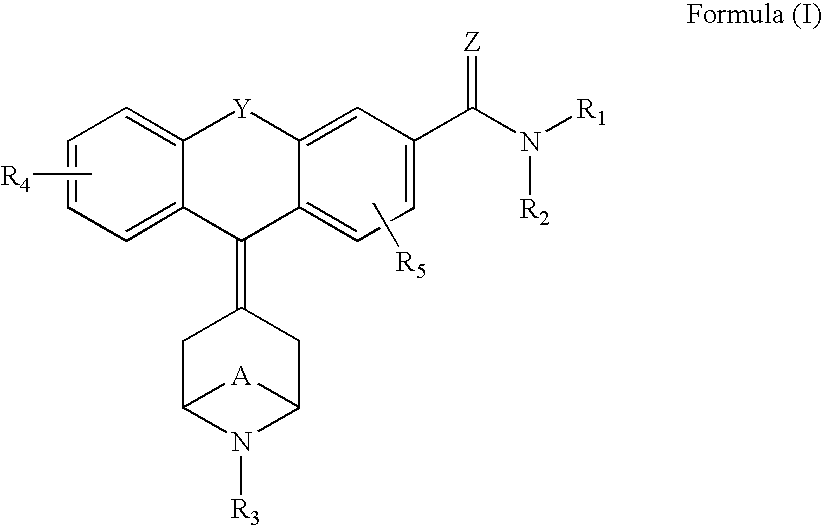
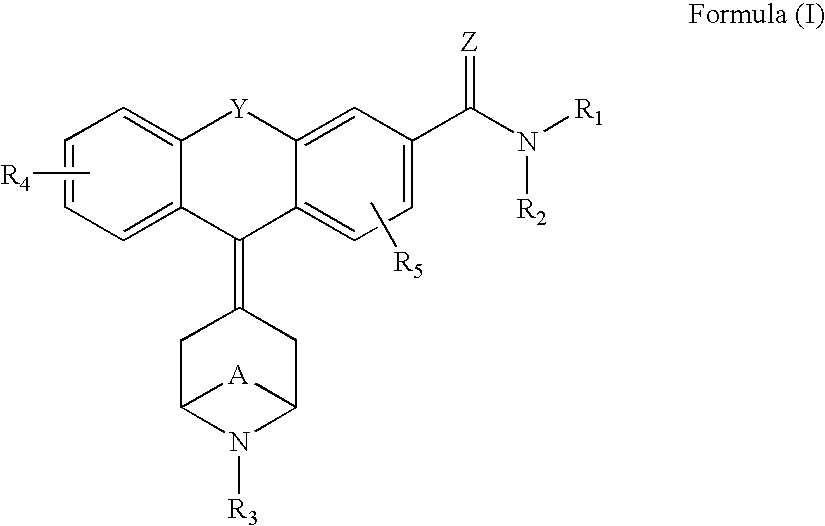
1-aryl-3-aminoalkoxy pyrazoles as sigma ligands enhancing analgesic effect of opioids and attenuating the dependency thereof
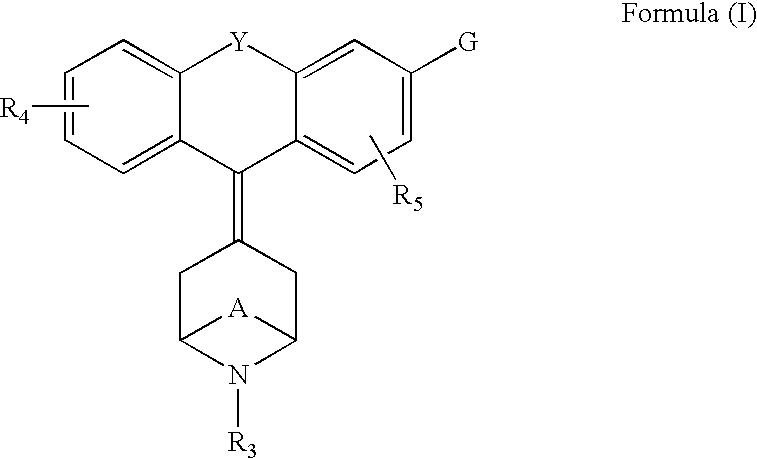
The invention relates to the use of a group of sigma receptor ligands of formula (I)for the potentiation of the analgesic effect of opioids and opiates and at the same time for decreasing the dependency induced by them.
Owner:LAB DEL DR ESTEVE SA
Tricyclic-bridged piperidinylidene derivatives as 8-opioid modulators
Owner:JANSSEN PHARMA NV
Enantiomerically pure opioid diarylmethylpiperazine as a cardioprotection agent
(−)3-((S)-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)(3-thienyl)methyl)phenol and pharmaceutically acceptable esters or salts thereof, in essentially enantiomerically pure form have utility as a cardioprotection agent.
Owner:DMK PHARM CORP +1
Sigma ligands for potentiating the analgesic effect of opioids and opiates in post-operative pain and attenuating the dependency thereof
The invention refers to a combination comprising a sigma ligand of formula (I) and an opioid or opiate for use in the prevention and / or treatment of pain developed as a consequence of surgery, especially peripheral neuropathic pain, allodynia, causalgia, hyperalgesia, hyperesthesia, hyperpathia, neuralgia, neuritis or neuropathy. The invention also refers to the sigma ligands of formula (I) for use in potentiating the analgesic effect of an opioid or opiate and / or for decreasing the dependency induced thereby when said opioid or opiate is used in the prevention and / or treatment of pain developed as a consequence of surgery.
Owner:LAB DEL DR ESTEVE SA
Opioid delivery system
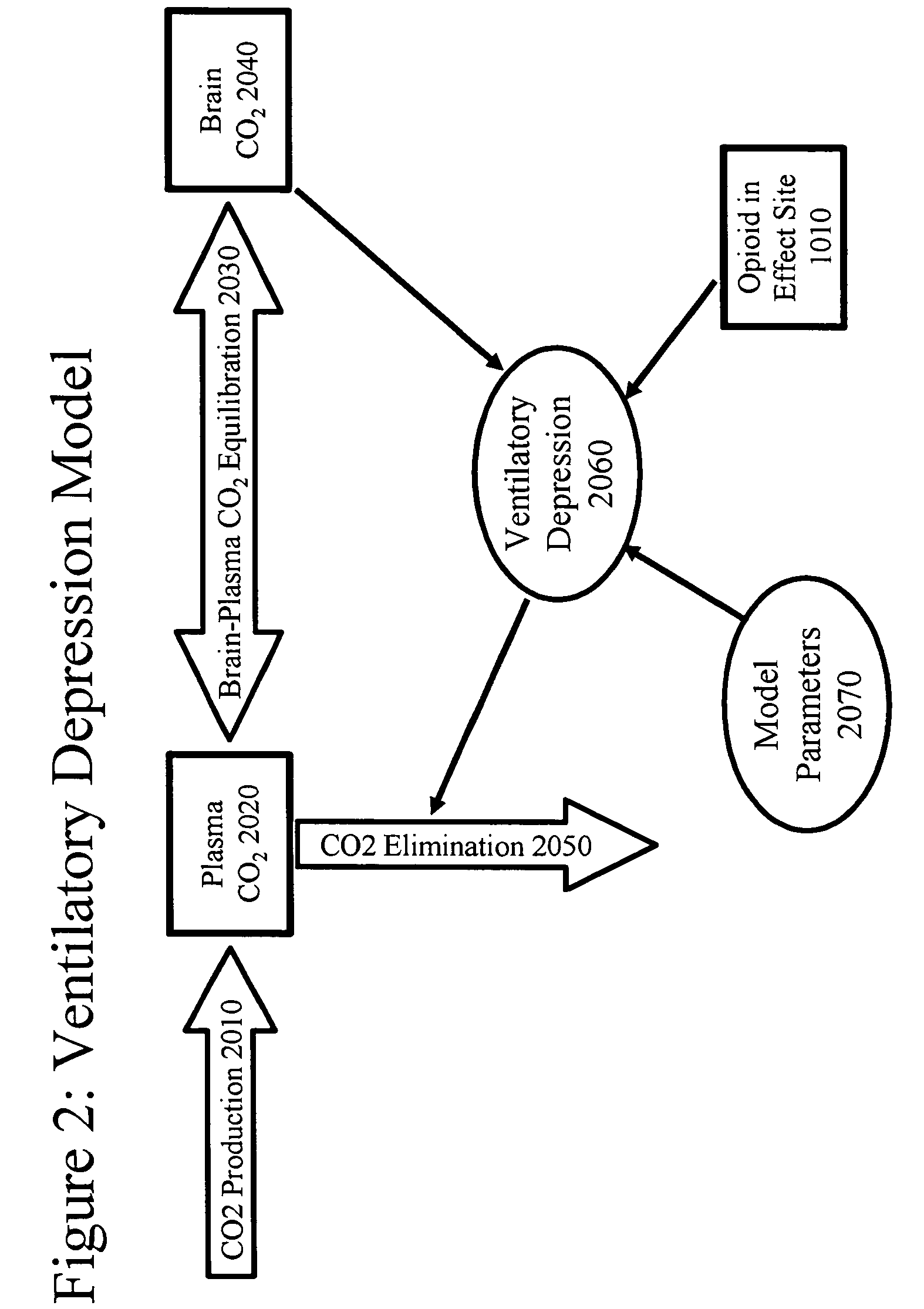
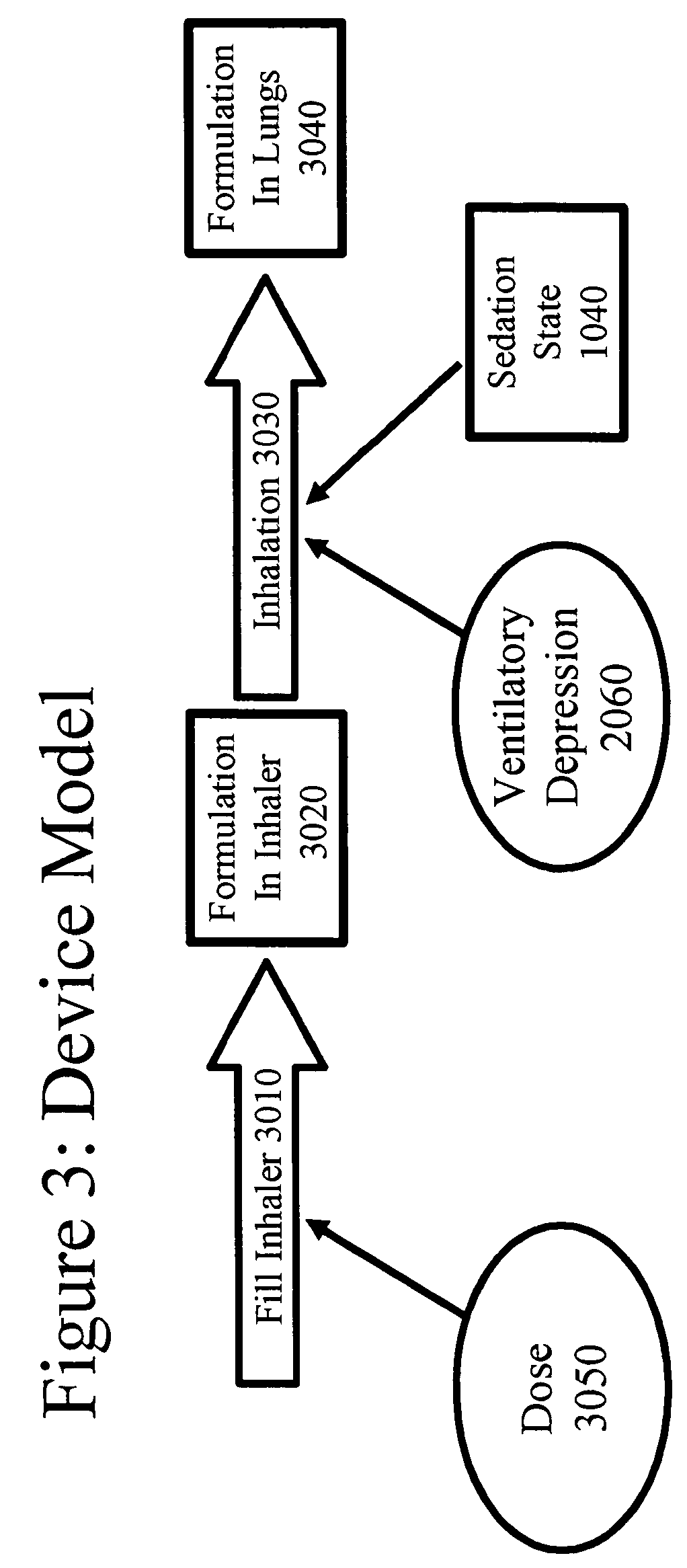
An opioid formulation for pulmonary administration in the treatment or management of pain, a pulmonary drug delivery device containing, method of administering, kit containing, and uses of same. The formulation contains at least one rapid-onset opioid and preferably also contains a sustained-effect opioid to reduce the frequency of administration. The invention employs the side effects of the opioid formulation to permit patients to self-limit drug intake, thereby avoiding toxicity while achieving analgesia. A pharmacokinetic and pharmacodynamic model is employed to determine optimum drug formulations and optimum parameters for administration.
Owner:YM BIOSCI
Non-invasive method for prediction of opioid-analgesia and opioid-blood-concentrations
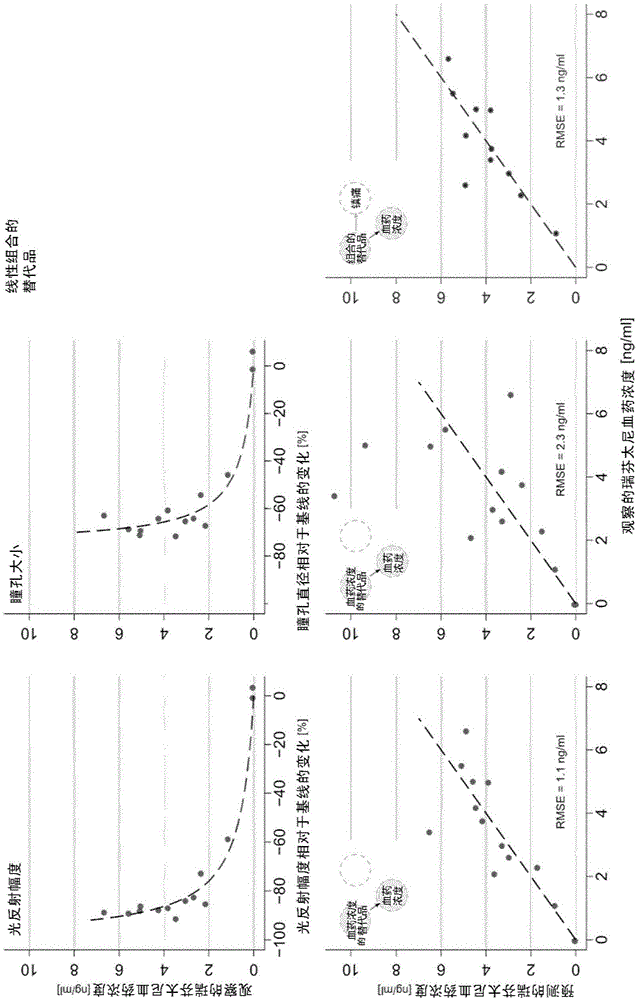
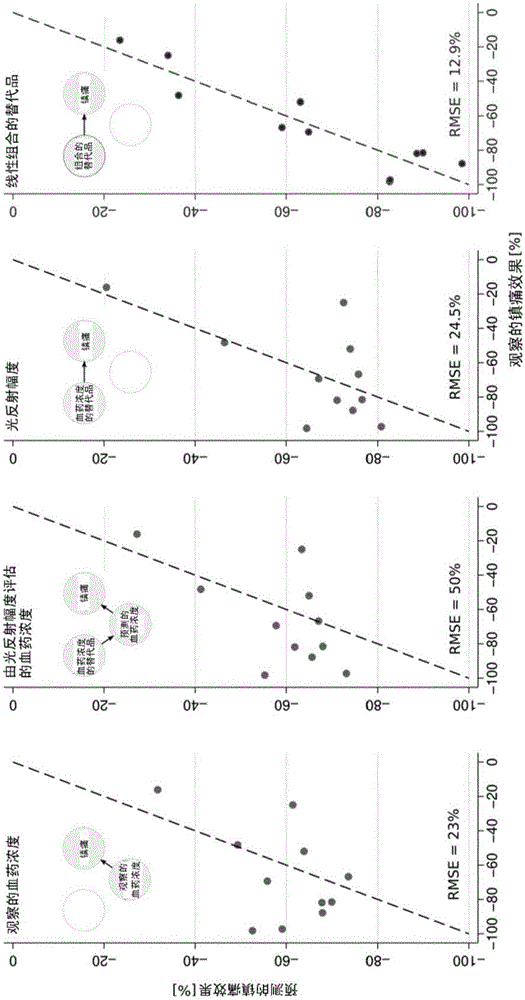
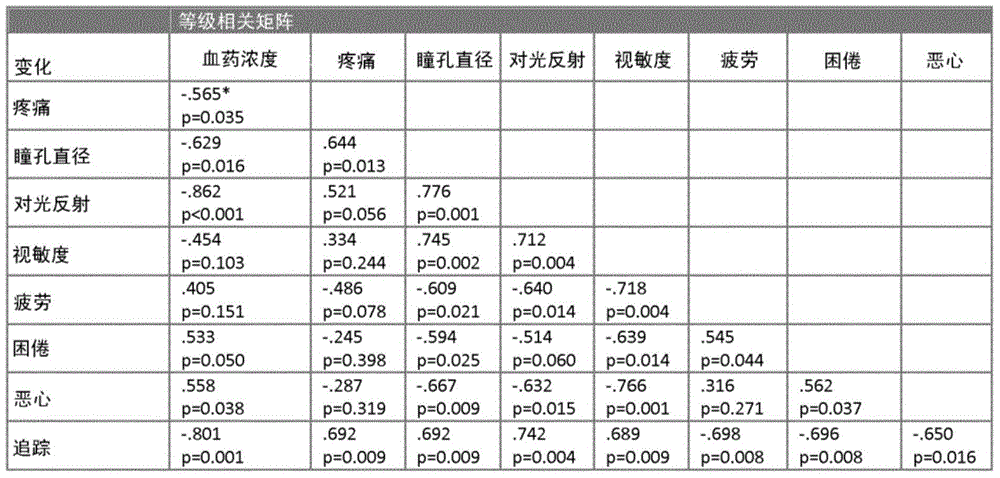
The present invention pertains to a non-invasive method and apparatus for predicting or monitoring analgesia and blood levels of opioid drugs in a patient receiving pain treatment, e.g., during palliative treatment. The inventive method comprises the measurement of one or more, preferably two or more, surrogate markers of a patient. According to the present invention surrogate markers correlate with the level of analgesia in the opioid receiving subject and thus provide a non-invasive method to predict and monitor analgesia during a treatment. Even more, surrogate markers were identified which correlate with the blood-concentration of the opioid in the subject. Thus, the invention provides a valuable clinical tool to assess and control pain treatments with opioids. Disclosed is the prediction method, an apparatus suitable for performing the inventive methods as well as the apparatus for use in medical treatments, such as pain therapy.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Mutant lactobacillus beta-glucuronidase enzymes with enhanced enzymatic activity
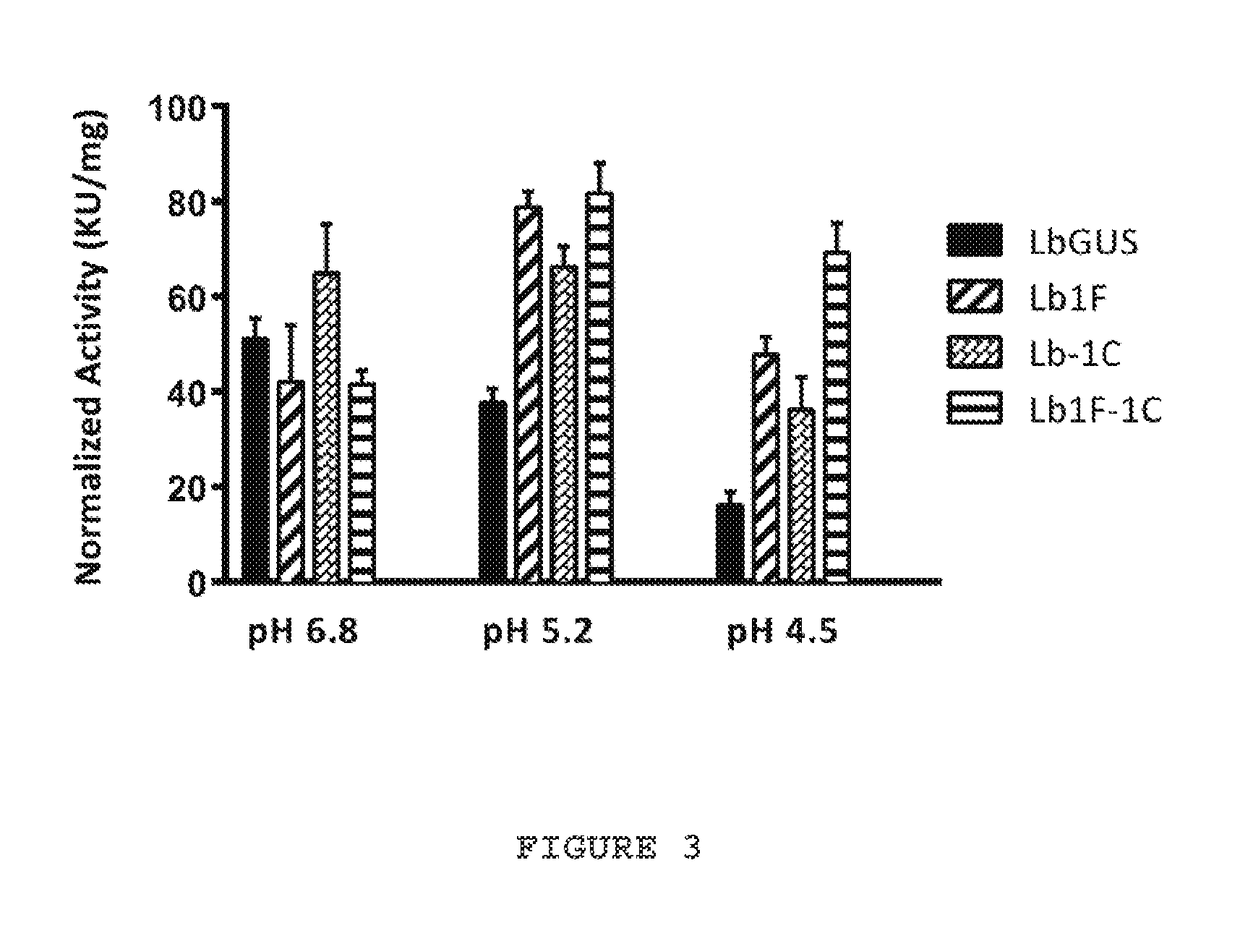
ActiveUS20170267985A1Increased enzymatic activityHigh thermal stabilityMicrobiological testing/measurementBiological material analysisD-GLUCURONIC ACIDDrug
Mutated Lactobacillus brevis strain 269Y β-glucuronidase enzymes with enhanced enzymatic activity at low pH (e.g., below pH 6.8), as well as enhanced thermostability as compared to wild type enzyme are provided. The enzymes of the invention advantageously allow for accurate analysis of bodily samples for the presence of drugs at low pH and in 30 minutes or less, as compared to the several hours needed using prior enzyme preparations. Methods of using the mutated enzymes for hydrolysis of glucuronide substrates, including opiates and benzodiazepines, are also provided.
Owner:INTEGRATED MICRO CHROMATOGRAPHY SYST INC
Abuse and misuse deterrent transdermal systems
Owner:4P THERAPEUTICS
Pharmaceutical composition for nasal delivery
ActiveUS10729687B1Improve bioavailabilityFast absorptionPowder deliveryOrganic active ingredientsOpioid antagonistOpioidergic
According to the invention, there is provided a solid pharmaceutical composition formulation for nasal delivery of an opioid antagonist, comprising a pharmacologically-effective amount of an opioid antagonist and a pharmaceutically-acceptable carrier. The compositions are preferably in the form of a powder produced by spray-drying, which are subsequently loaded into single use nasal applicators. Preferred pharmaceutically-acceptable carriers in this regard include disaccharides (e.g. lactose or trehalose) and dextrins (e.g. cyclodextrins or maltodextrins), preferably spray-dried together in combination. Compositions may further comprise an alkyl saccharide, preferably a sucrose ester, such as sucrose monolaurate. The compositions and applicators may be employed in the treatment of opioid overdose in subjects.
Owner:OREXO AB
Process for improved opioid synthesis
ActiveUS10316042B2More efficientMore volumeOrganic active ingredientsNervous disorderOpioidergicOxymorphone
Compounds and compositions for use as starting materials or intermediate materials in the preparation of opioids including, e.g., oxymorphone base and / or an oxymorphone salt; processes for preparing these compounds and compositions; uses of these compounds and compositions in the preparation of APIs and pharmaceutical dosage forms; and uses of said APIs and pharmaceutical dosage forms in the treatment of medical conditions.
Owner:NORDBOTICS INC
Liquid naloxone spray
InactiveCN109922805AOrganic active ingredientsPharmaceutical delivery mechanismOpioidergicOpioid overdose
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt, or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence-, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com