Abuse and misuse deterrent transdermal systems
a technology of abuse and misuse, which is applied in the direction of bandages, dressings, drug compositions, etc., can solve the problems of patch misuse, accidental misuse, severe adverse reactions,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
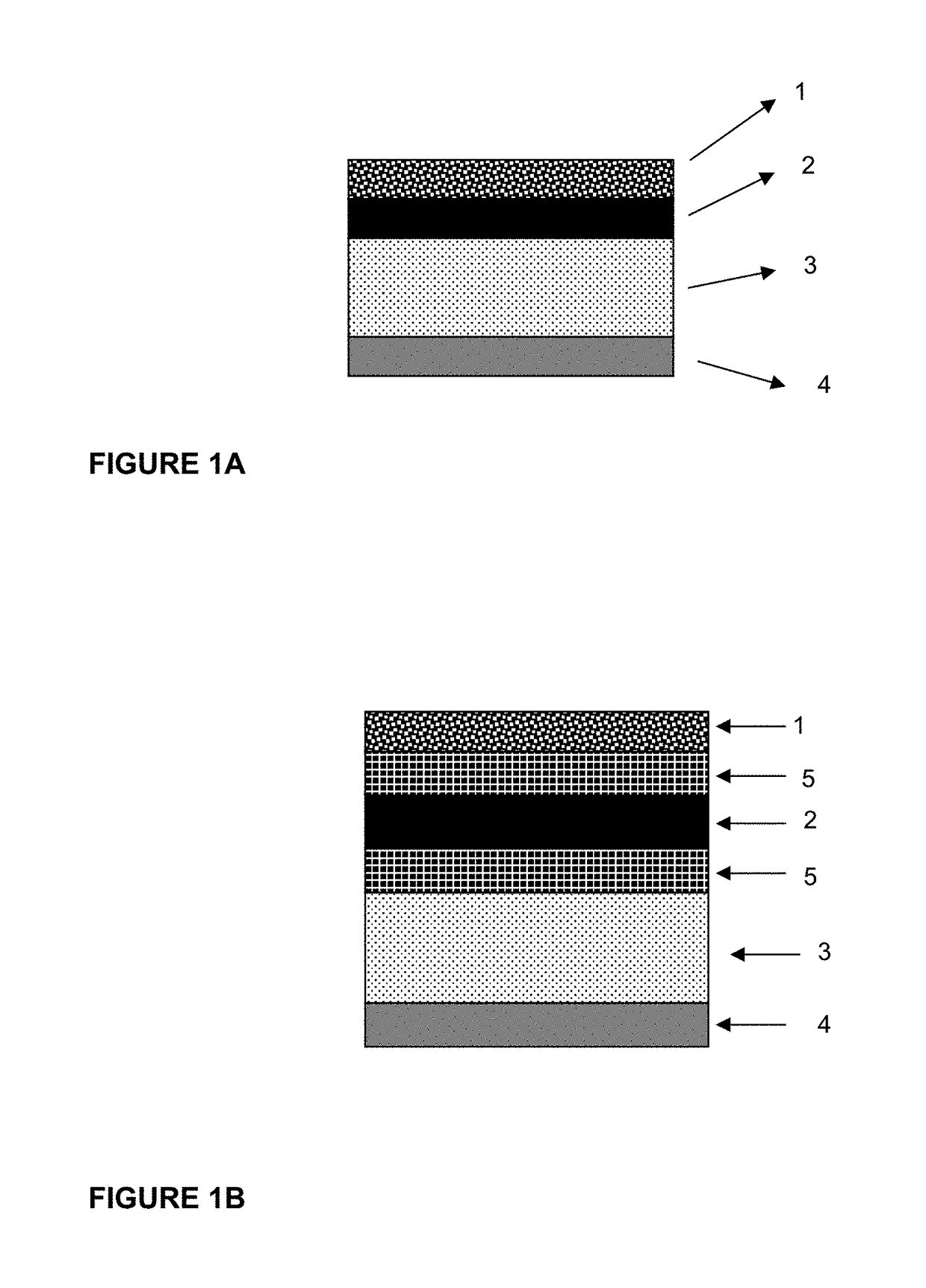
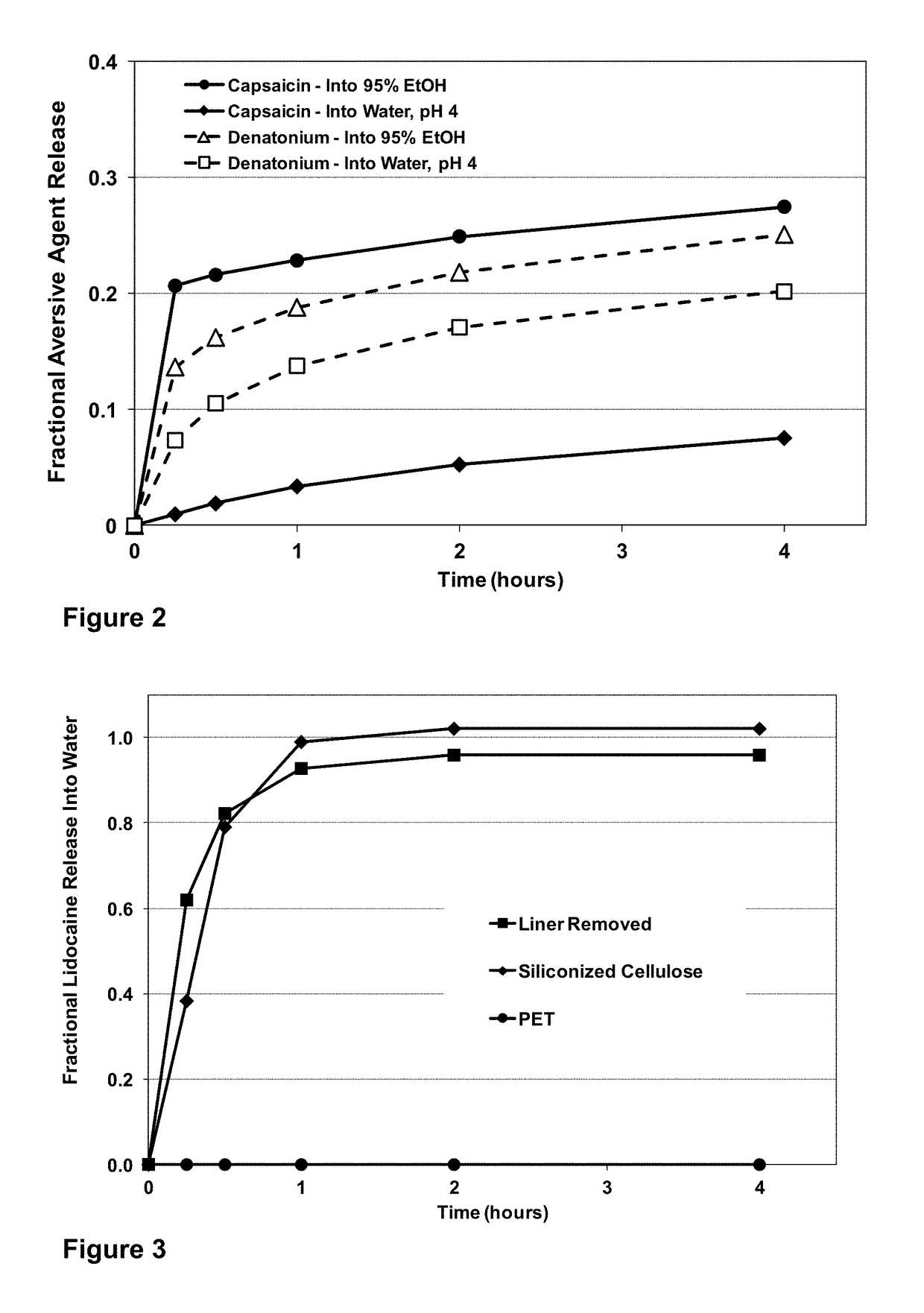
[0040]An abuse deterrent system backing for a transdermal patch is prepared using a commercial backing comprising a 50 micrometer polyethylene terephthalate film bound to an ethylene / vinyl acetate film containing dissolved and dispersed aversive agents and aversive agents in dispersed poly(DL-lactide-co-glycolide) microparticles. A mixture of denatonium benzoate (1 weight percent),capsaicin (3 weight percent), and polyvinyl pyrrolidone (3 weight percent; K value 30) are added to ethylene / vinyl acetate copolymer (40 percent VA). The mixture is heated and agitated in the mixing chamber of a kneader extruder until uniform. A 1:3 blend of denatonium benzoate and capsaicin totaling 10 weight percent on a dry basis is dissolved in a 10 weight percent solution of poly(DL-lactide-co-glycolide) in methylene chloride. Microparticles of the aversive agents and PLGA are manufactured via spray drying or oil-in-water emulsion / evaporation and the particles are added at a 20 weight percent loading ...
example 2
[0042]An abuse deterrent system backing for a transdermal patch is prepared using a commercial backing comprising a 50 micrometer polyethylene terephthalate film bound to an ethylene / vinyl acetate film containing dissolved and dispersed aversive agent and aversive agent in dispersed poly DL-lactide microparticles. A mixture of capsaicin (5 weight percent), and polyvinyl pyrrolidone (3 weight percent; K value 30) are added to ethylene / vinyl acetate copolymer (40 percent VA). The mixture is heated and agitated in the mixing chamber of a kneader extruder until uniform. Capsaicin (10 weight percent) is dissolved in a 10 weight percent solution of poly DL-lactide in methylene chloride. Microparticles of capsaicin and poly DL-lactide are manufactured via spray drying or oil-in-water emulsion / evaporation and the particles are added at a 20 weight percent loading to the heated blend of aversive agent, polyvinylpyrrolidone and ethylene / vinyl acetate copolymer and the mix is agitated until un...
example 3
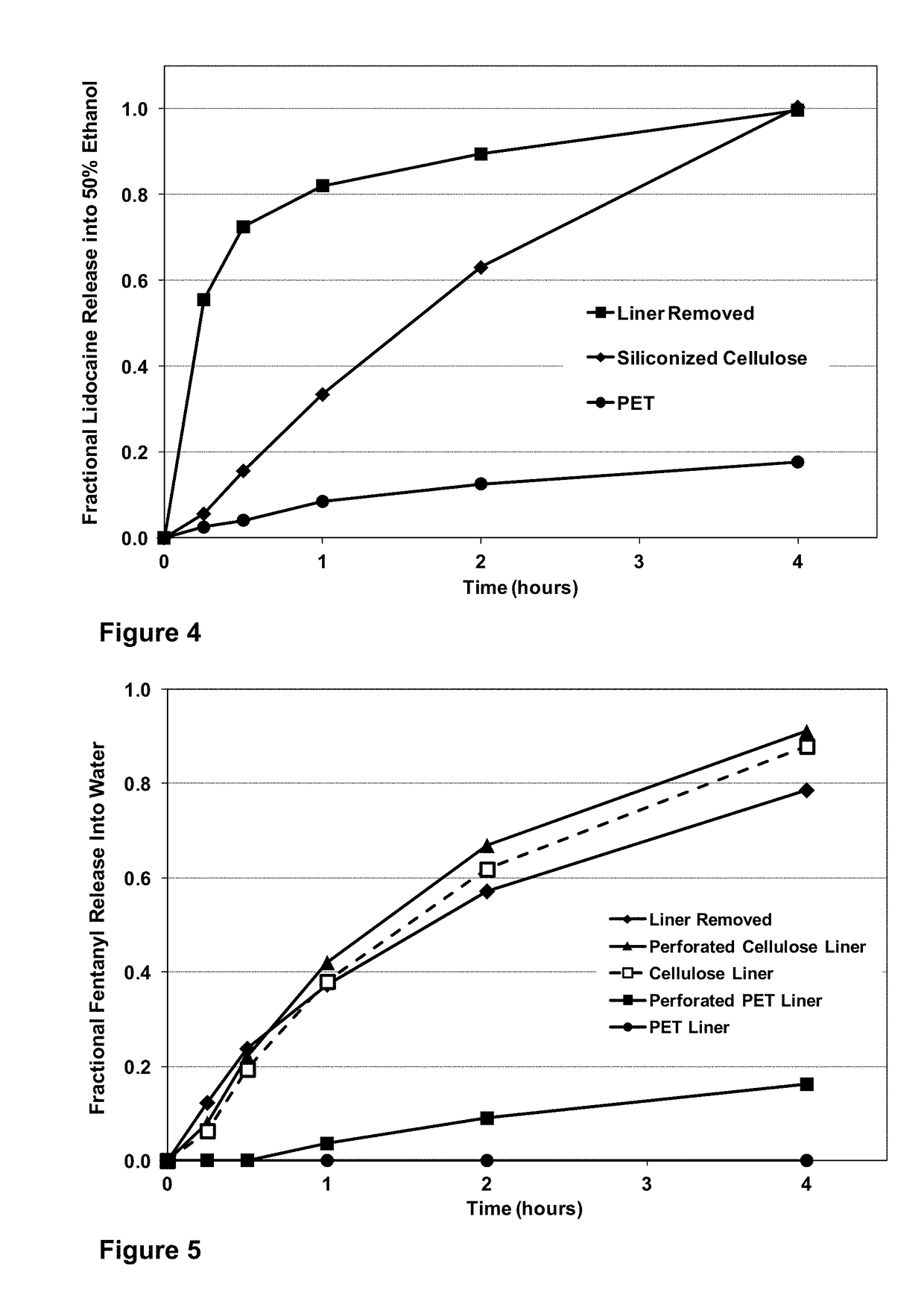
[0044]An abuse deterrent system backing for a transdermal patch is prepared using a commercial backing comprising a layer of non-woven polyester adhesively laminated to a layer of dense polyethylene terephthalate film. 2 weight percent denatonium benzoate and 5 weight percent capsaicin and 5 weight percent polyvinyl pyrrolidone (K value 30) are added (on a solids basis) to a 40 weight percent solution of ethylene / vinyl acetate copolymer (40 percent VA) dissolved in methylene chloride. The mixture is agitated until uniform and then cast into a film on the non-woven side of the commercial backing material and allowed to dry.
[0045]A standard formulation of fentanyl in acrylic adhesive solution used in common sub saturated fentanyl transdermal patches is cast on the dense polyethylene terephthalate film side of the backing and allowed to dry. A 2 mil thick film of hydratable cellulose coated with a silicone release coating is laminated to the dried adhesive fentanyl containing layer. Fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com