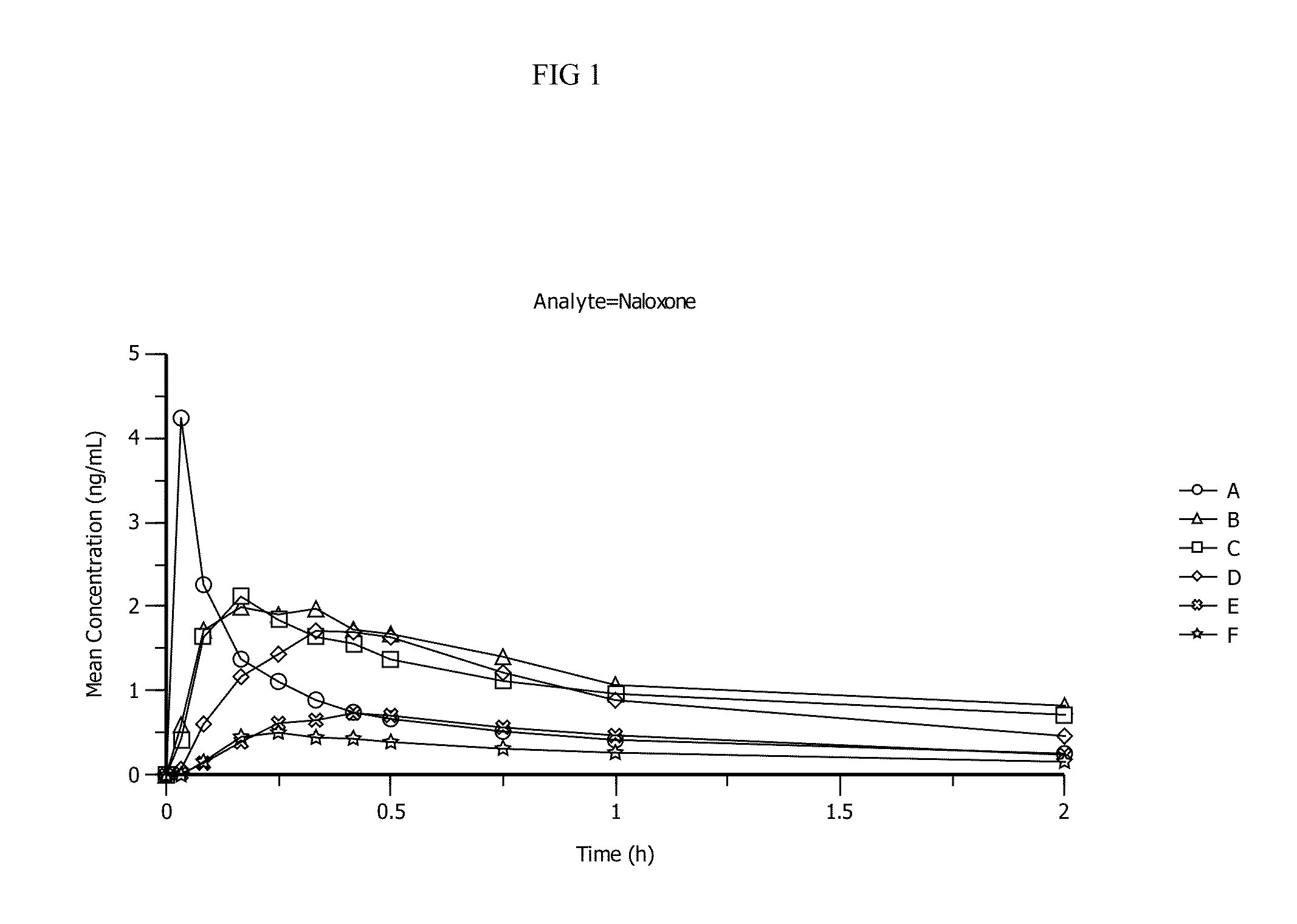
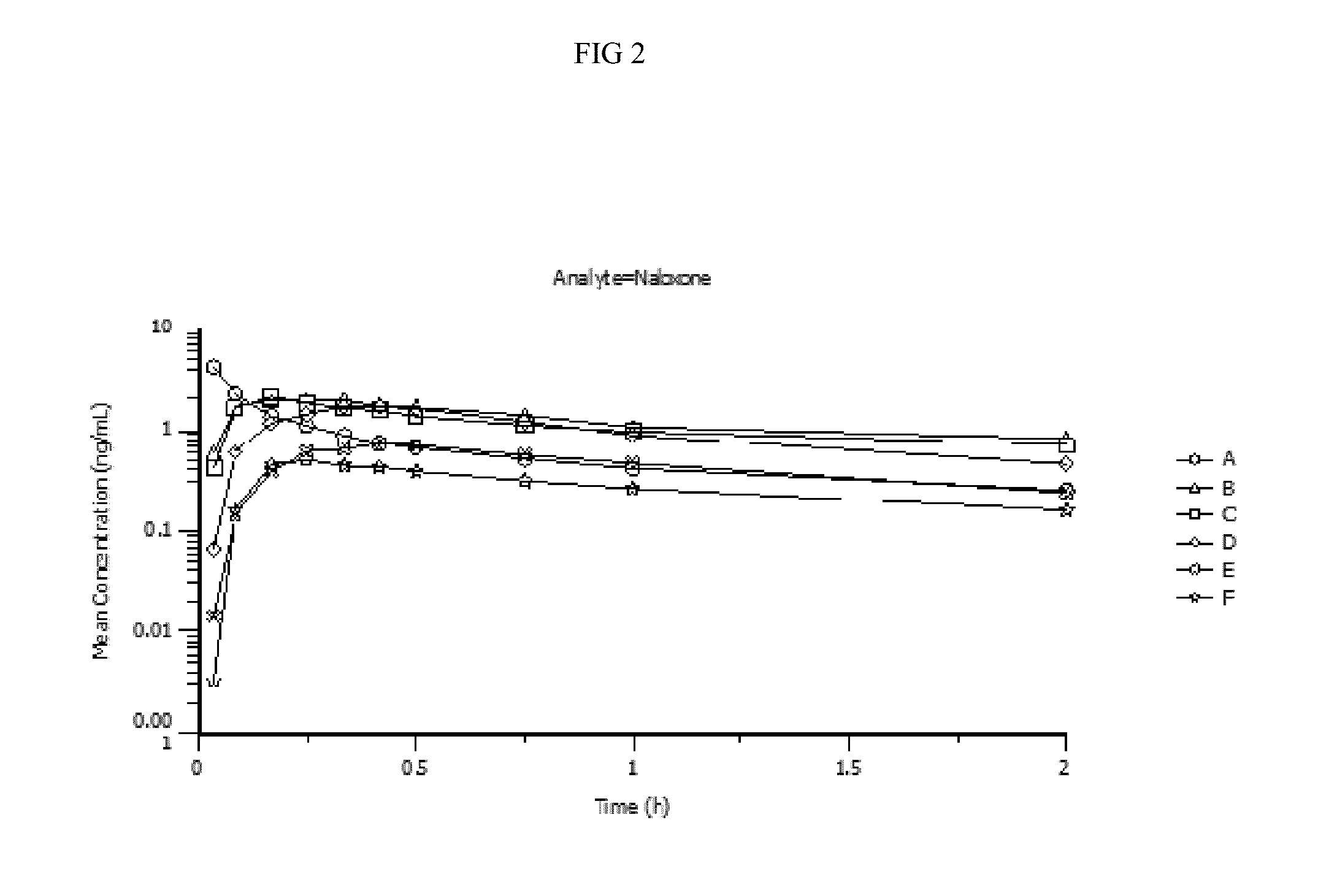
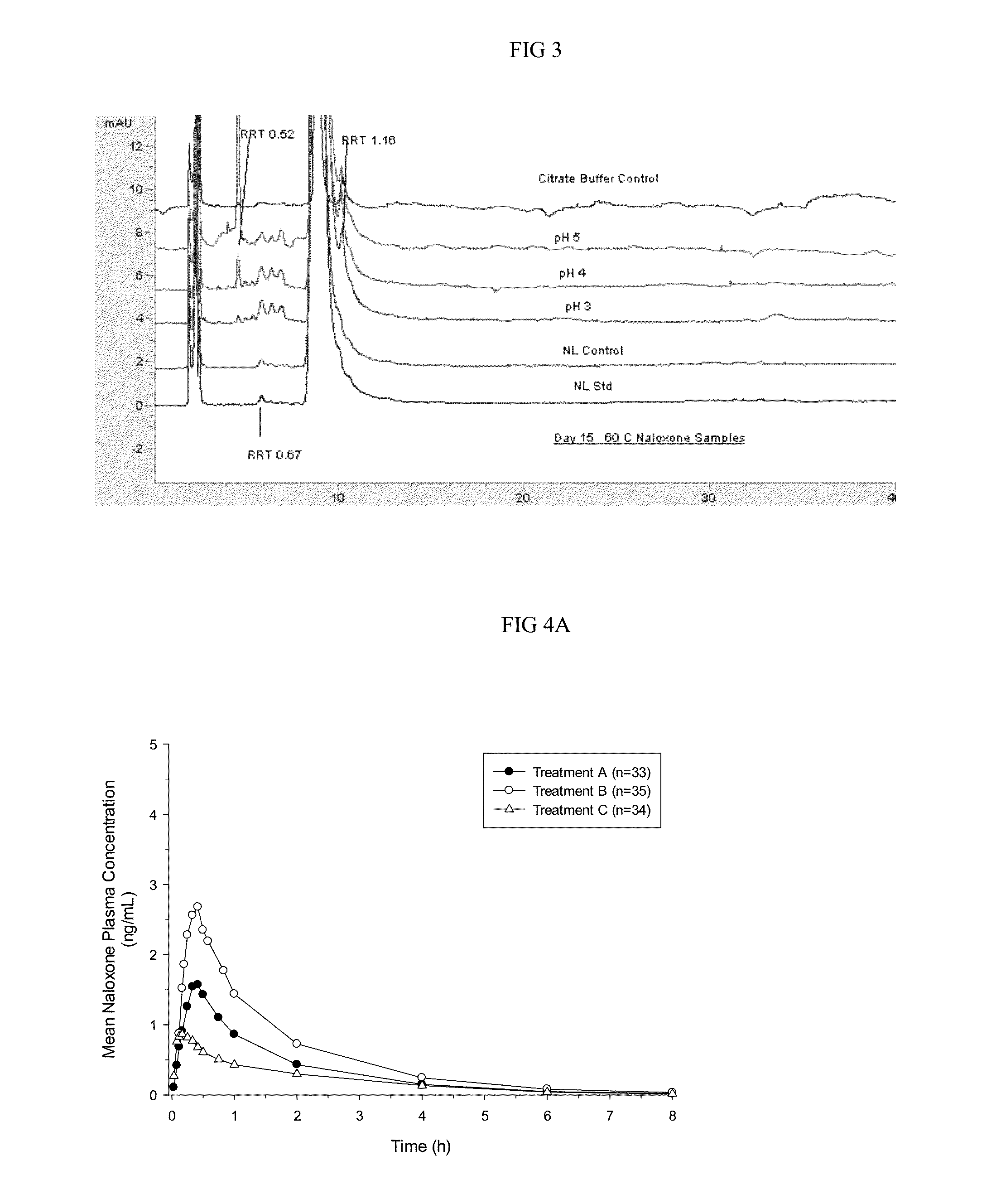
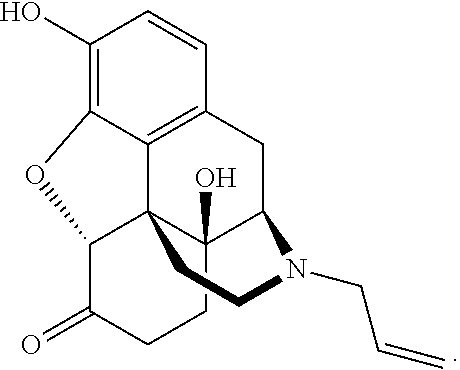
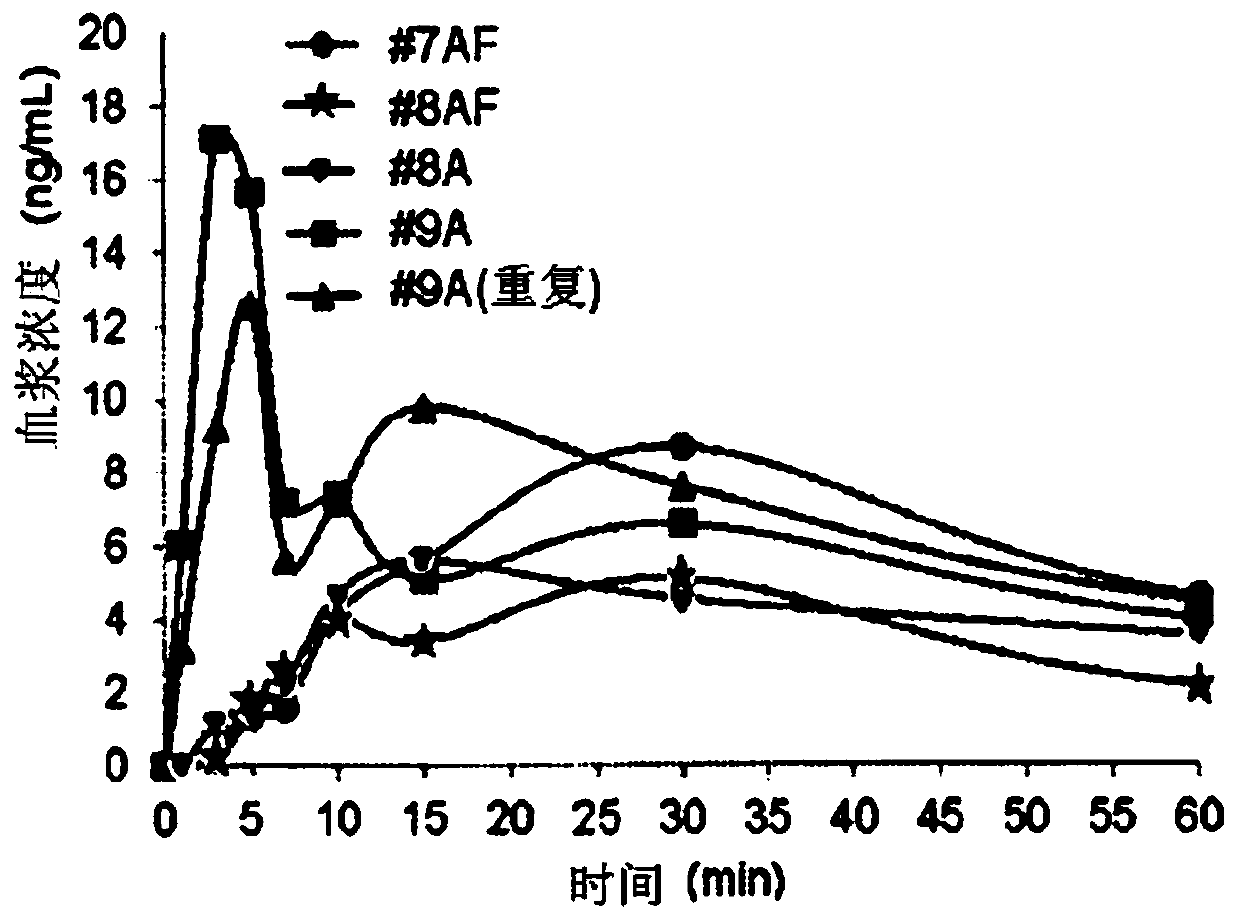
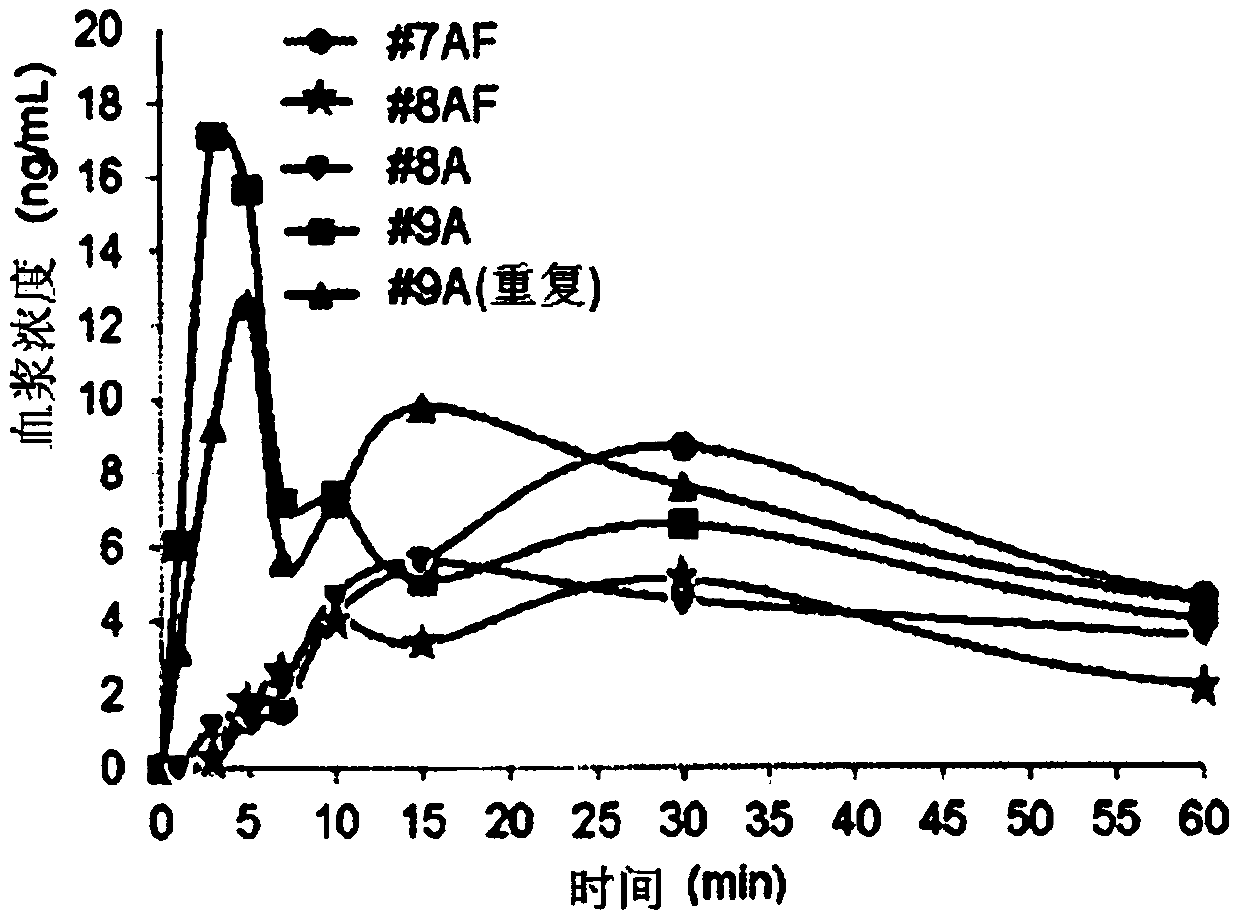
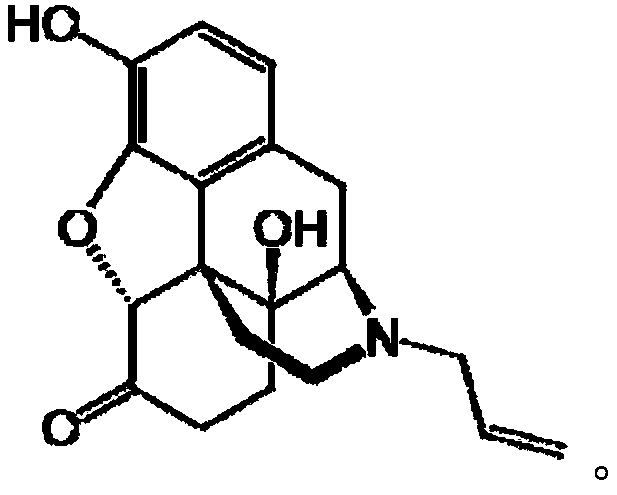
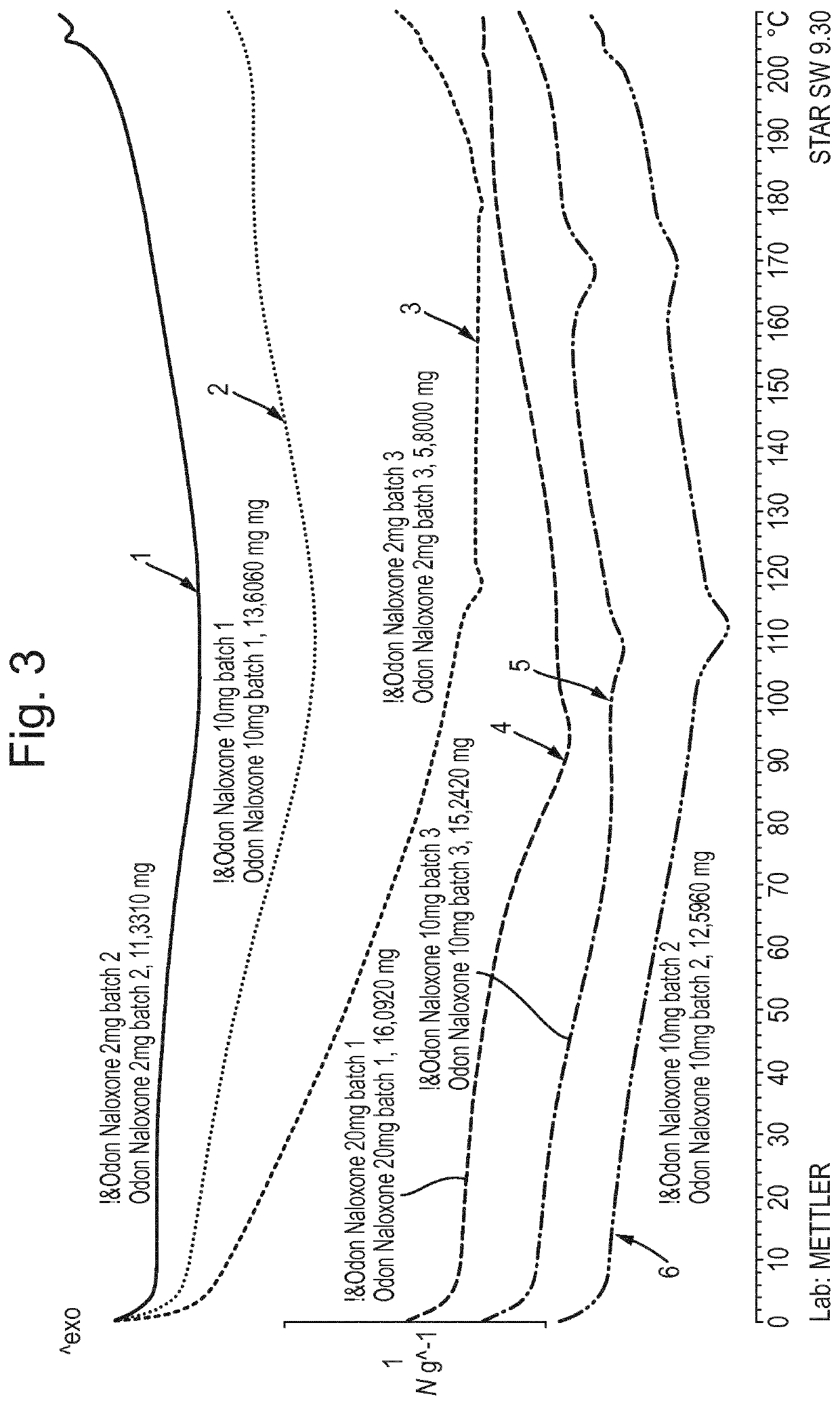
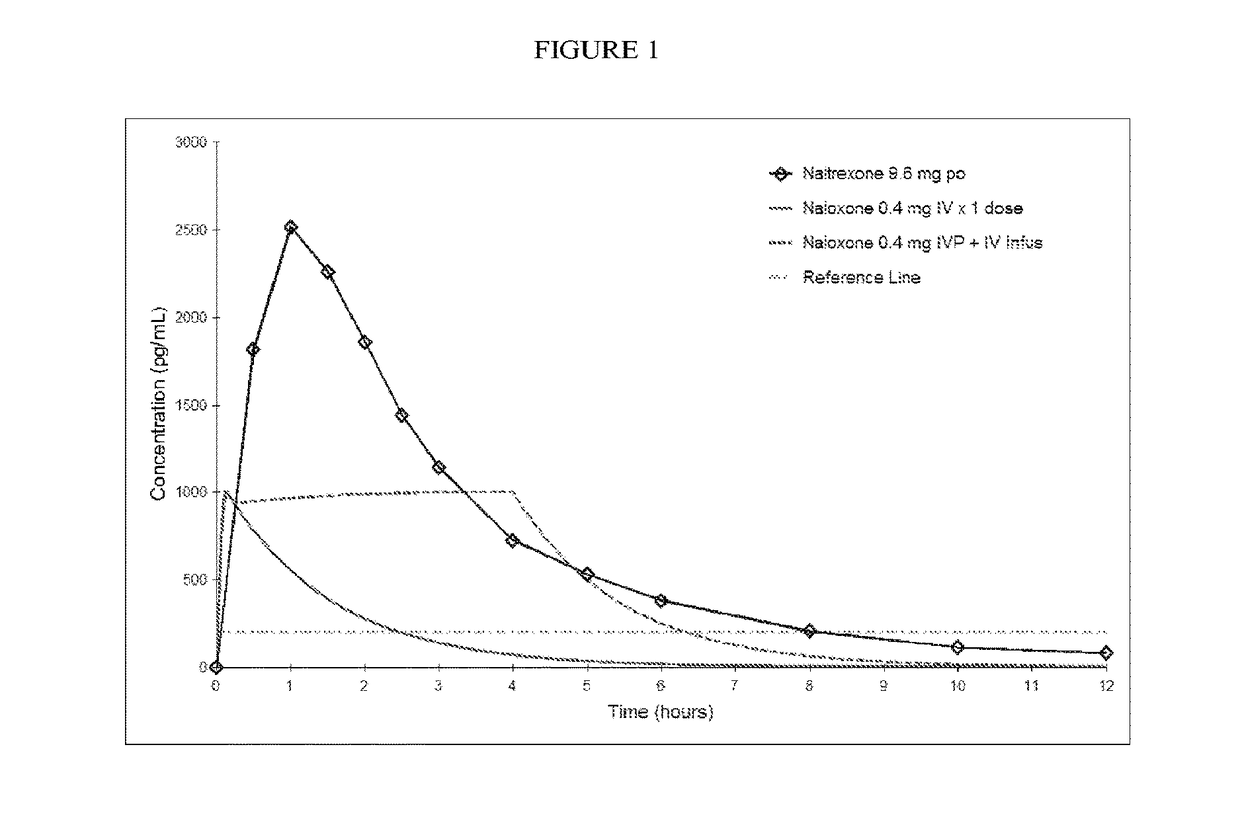
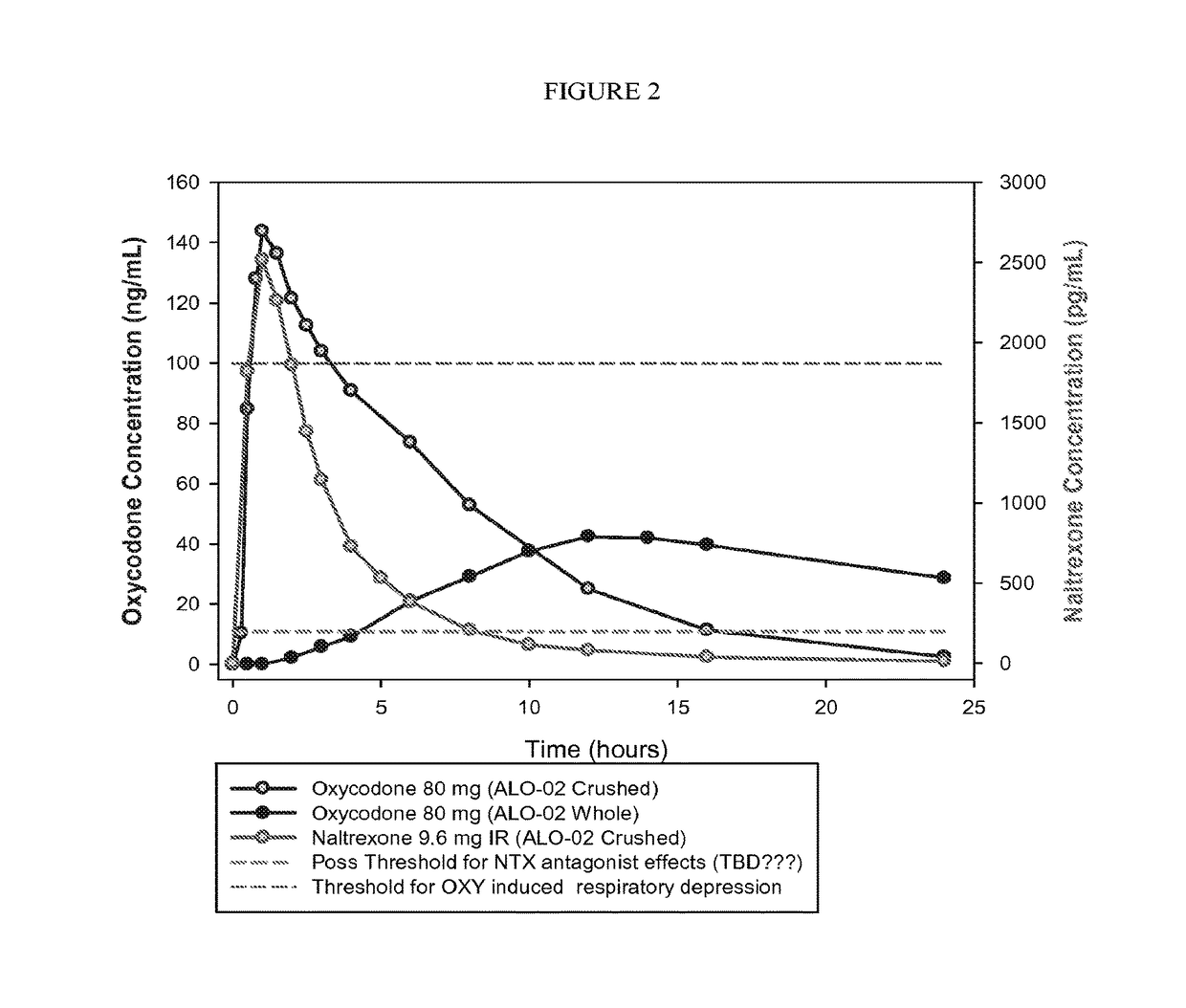
Patents
Literature
51 results about "Opioid overdose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
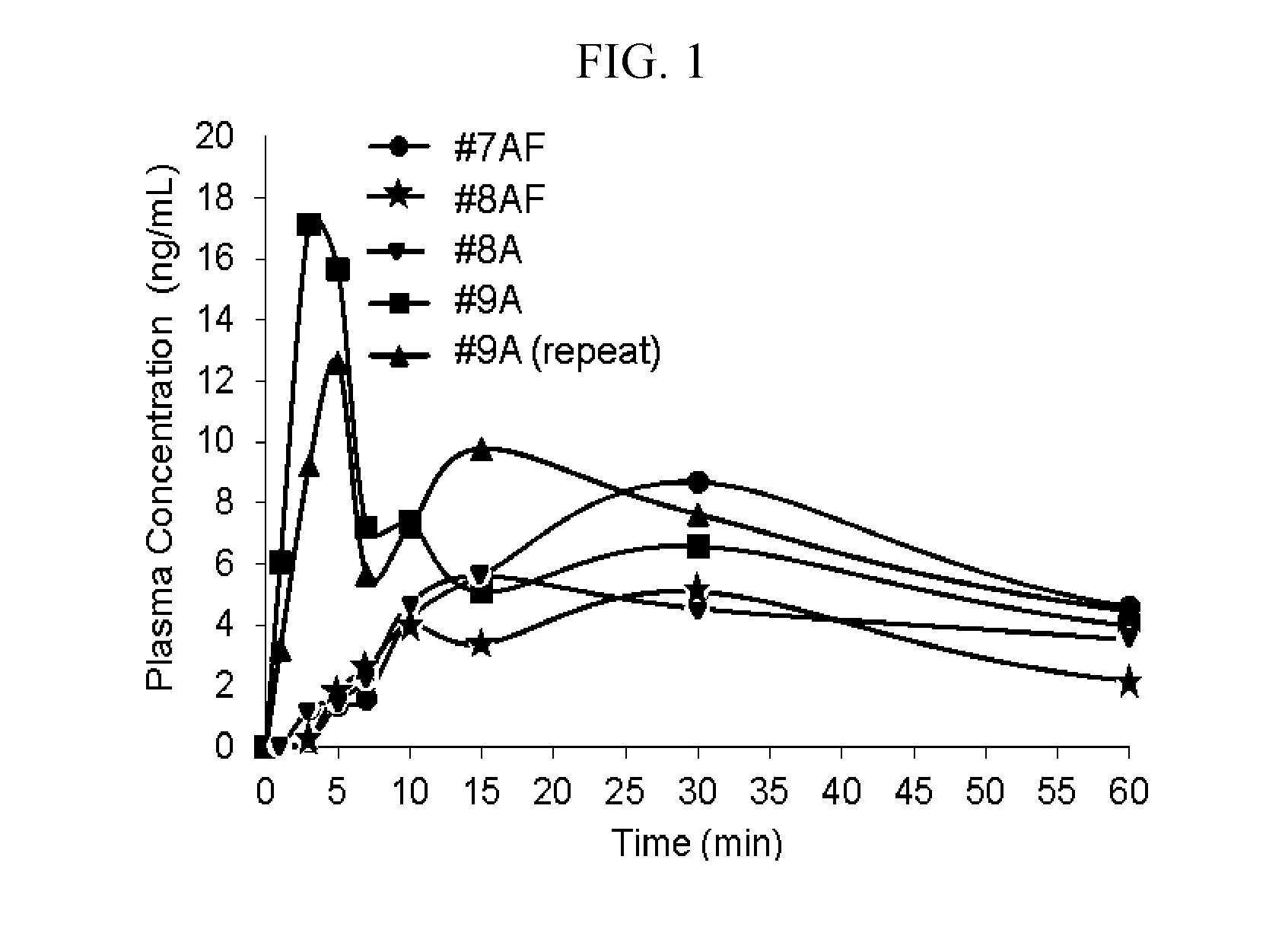
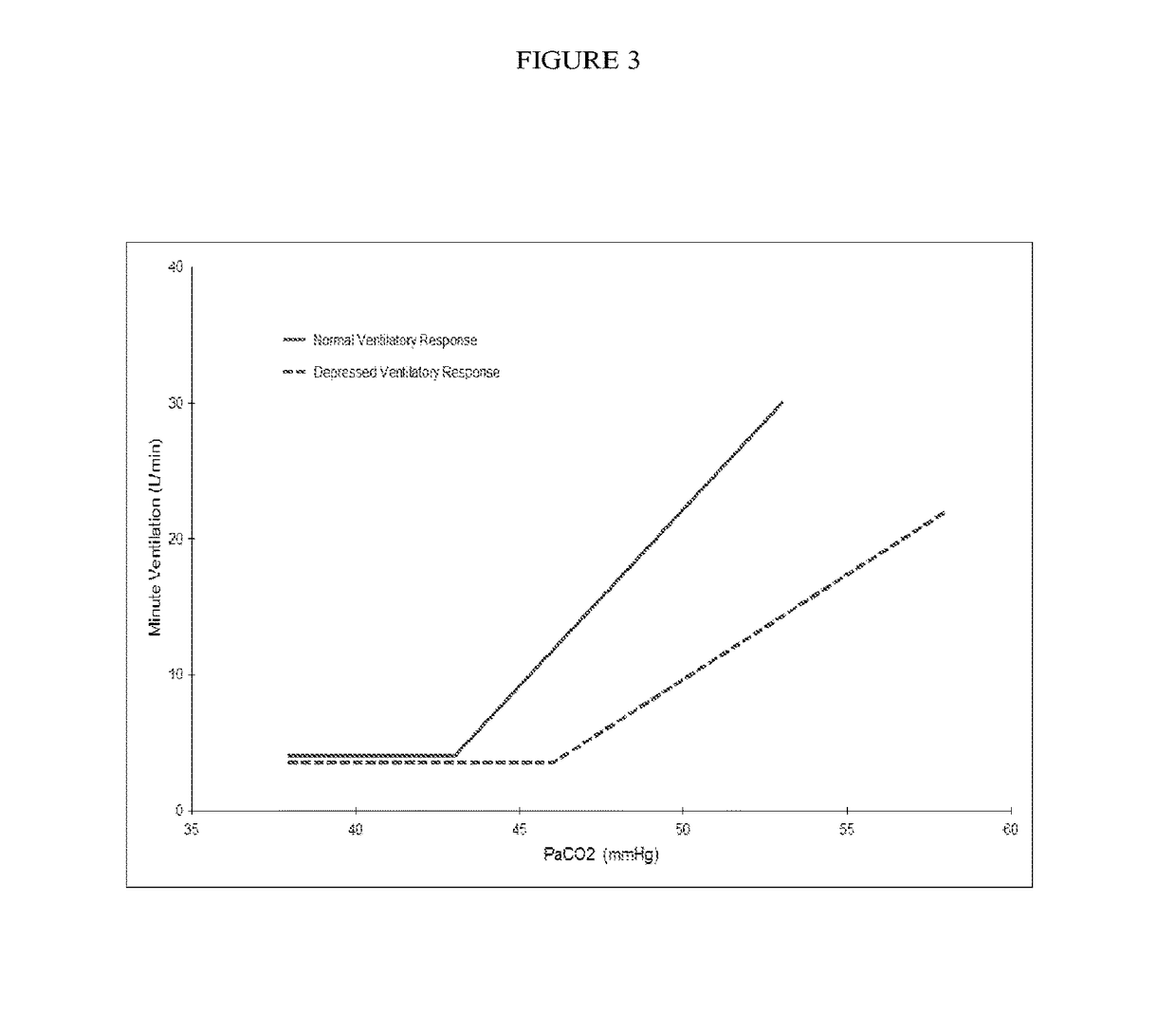
An opioid overdose is toxicity due to excessive opioids. Examples of opioids include morphine, heroin, fentanyl, tramadol, and methadone. Symptoms include insufficient breathing, small pupils, and unconsciousness. Onset of symptoms depends in part on the route opioids are taken. Among those who initially survive, complications can include rhabdomyolysis, pulmonary edema, compartment syndrome, and permanent brain damage.
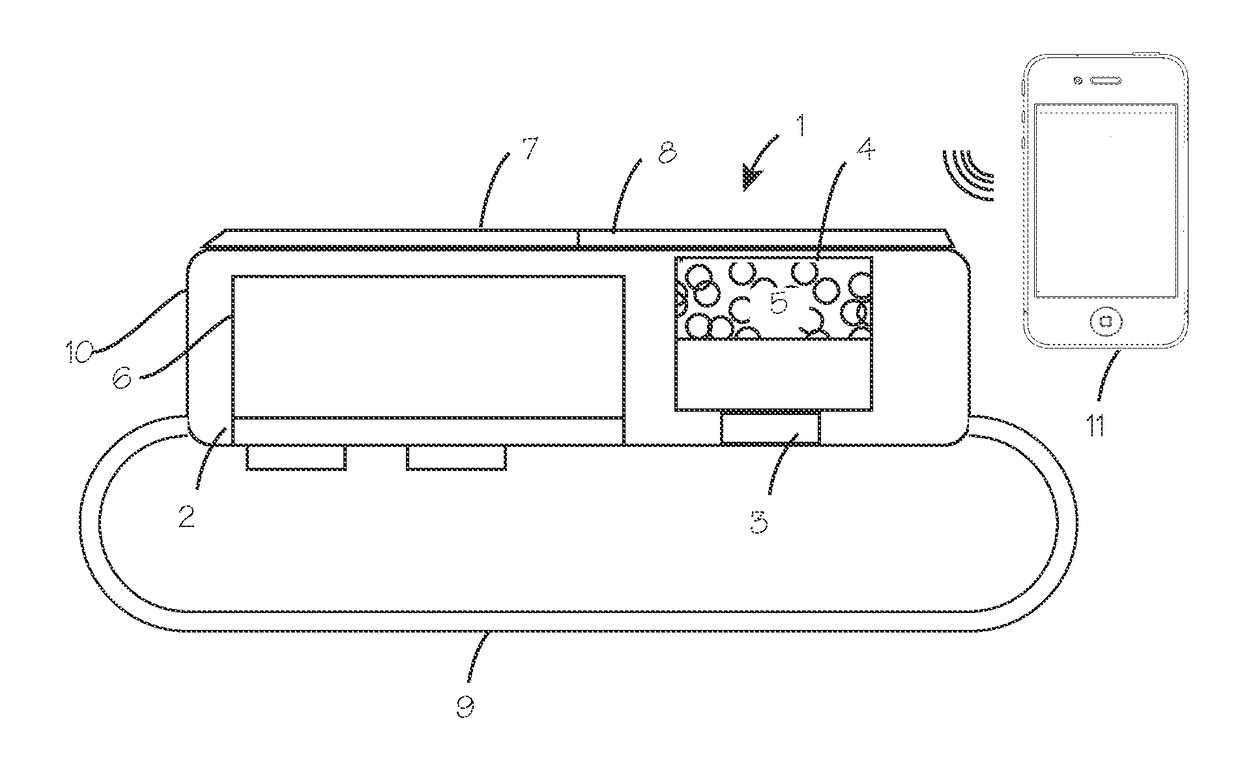
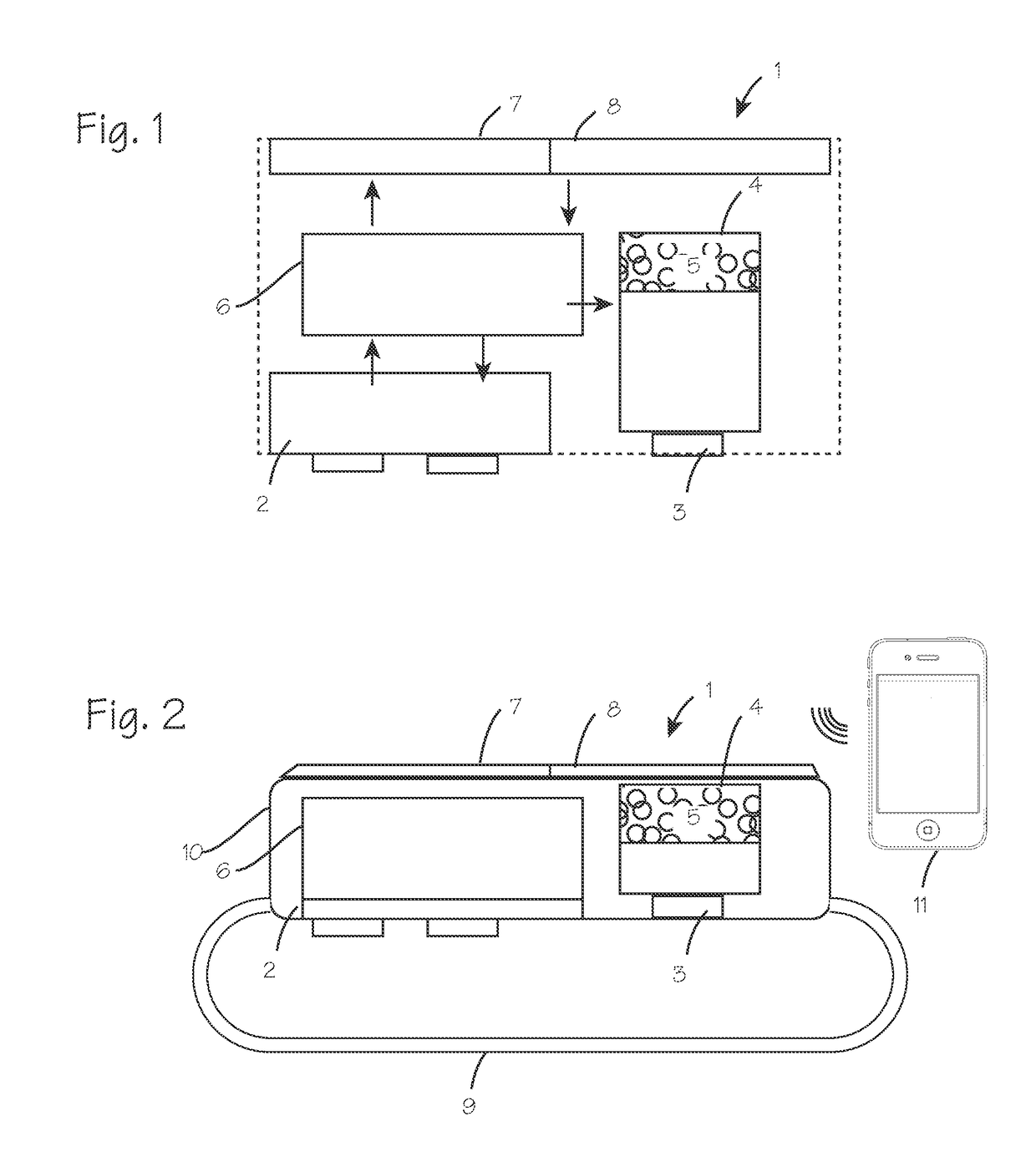
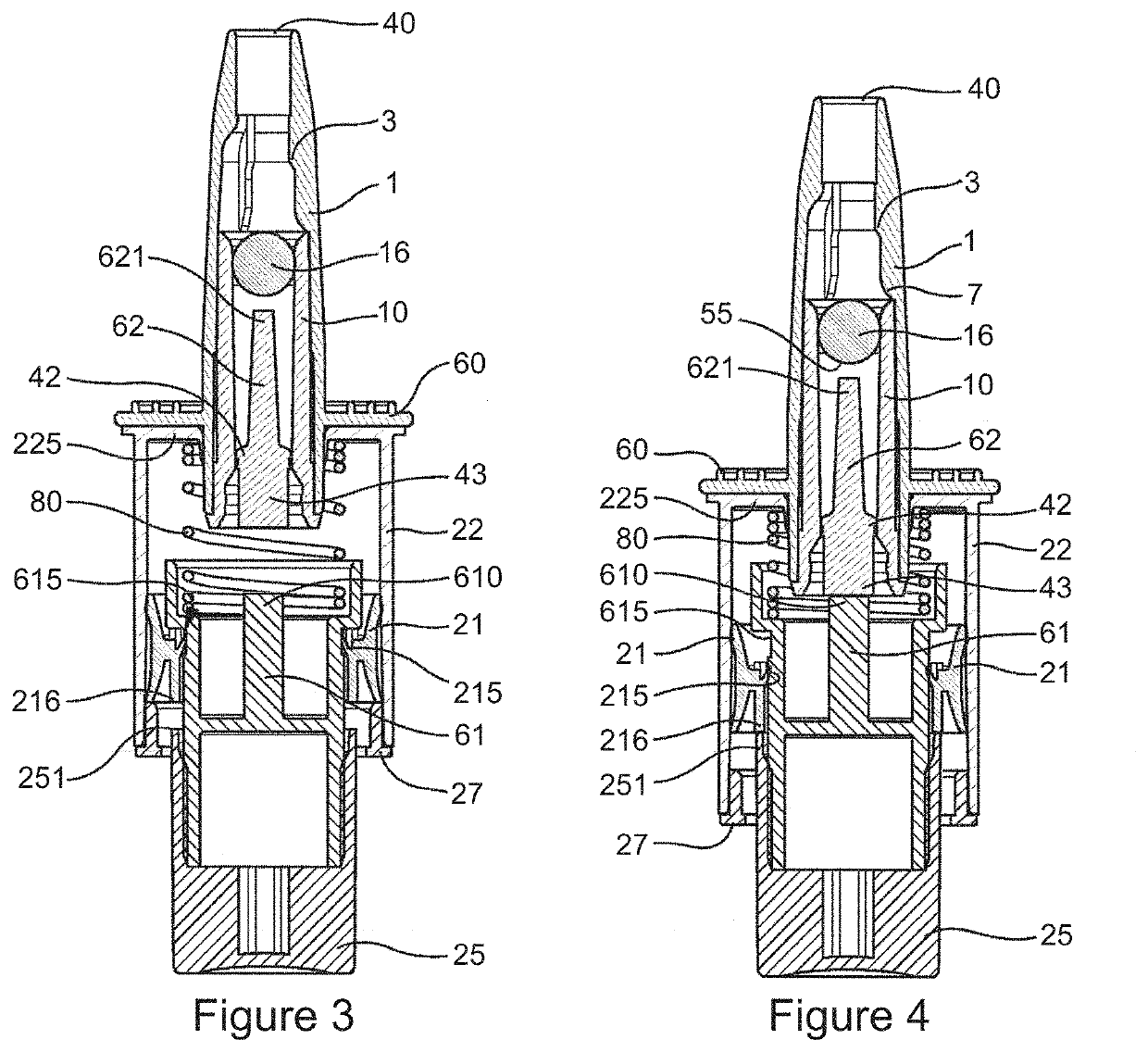
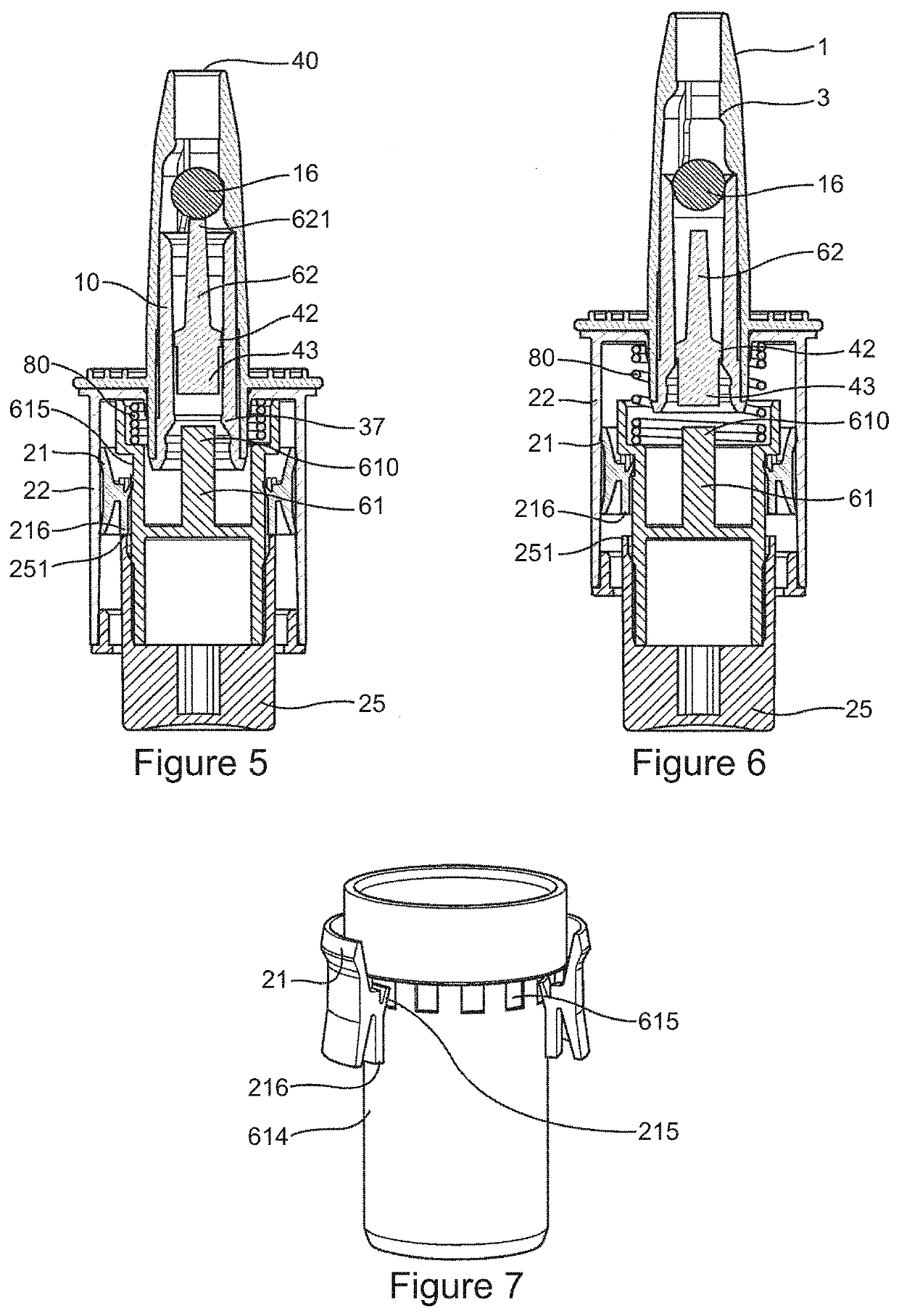
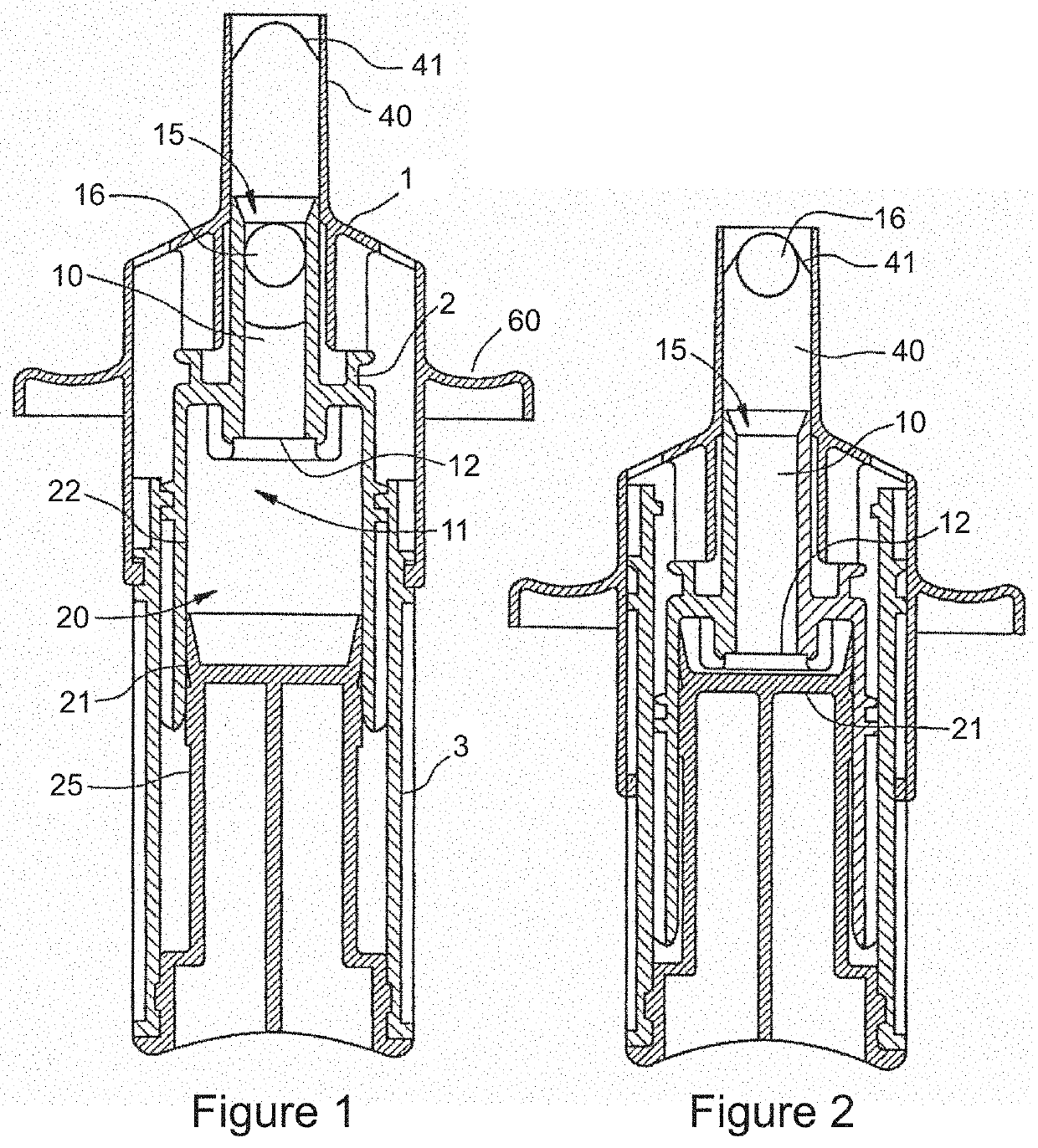
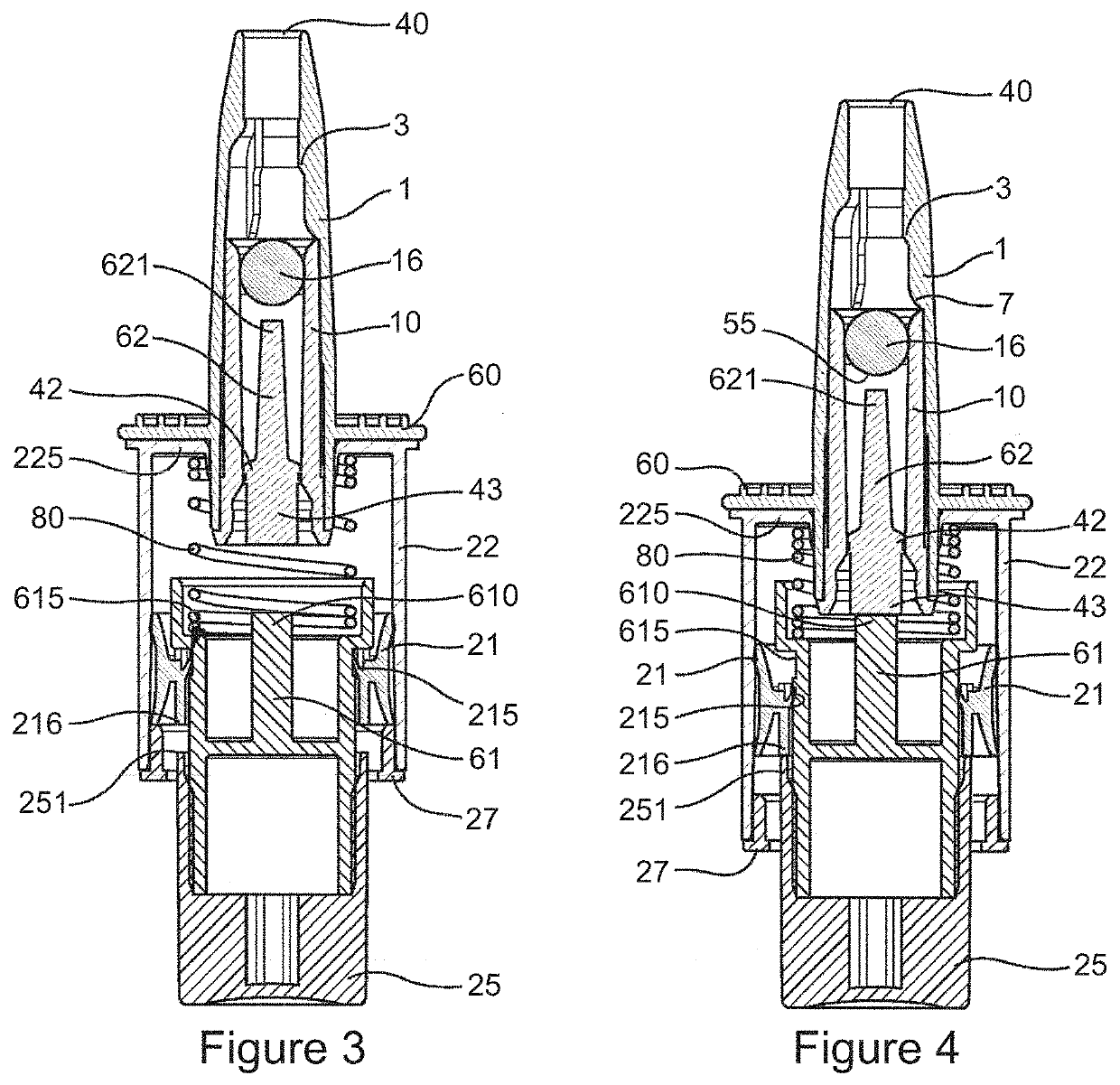
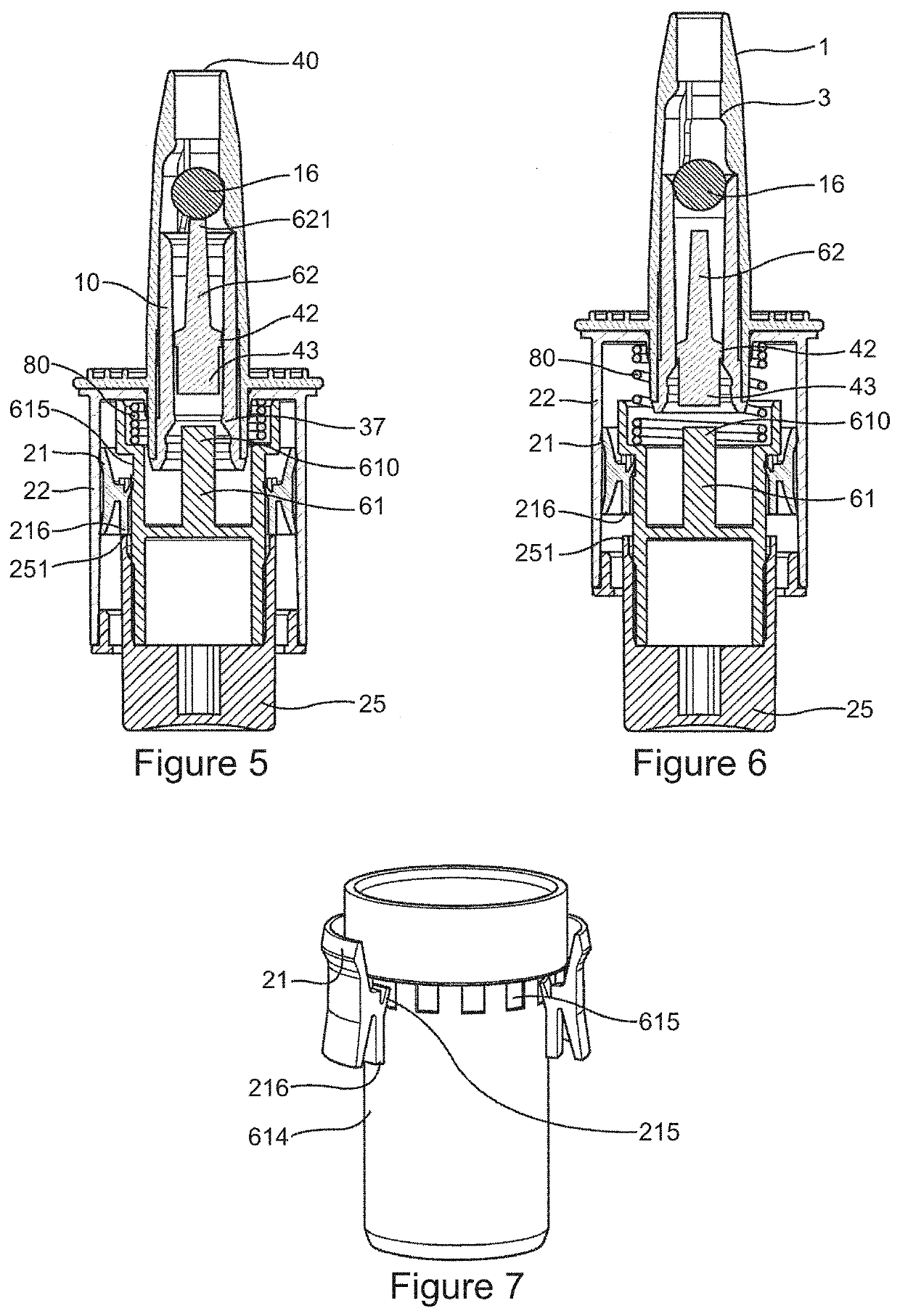
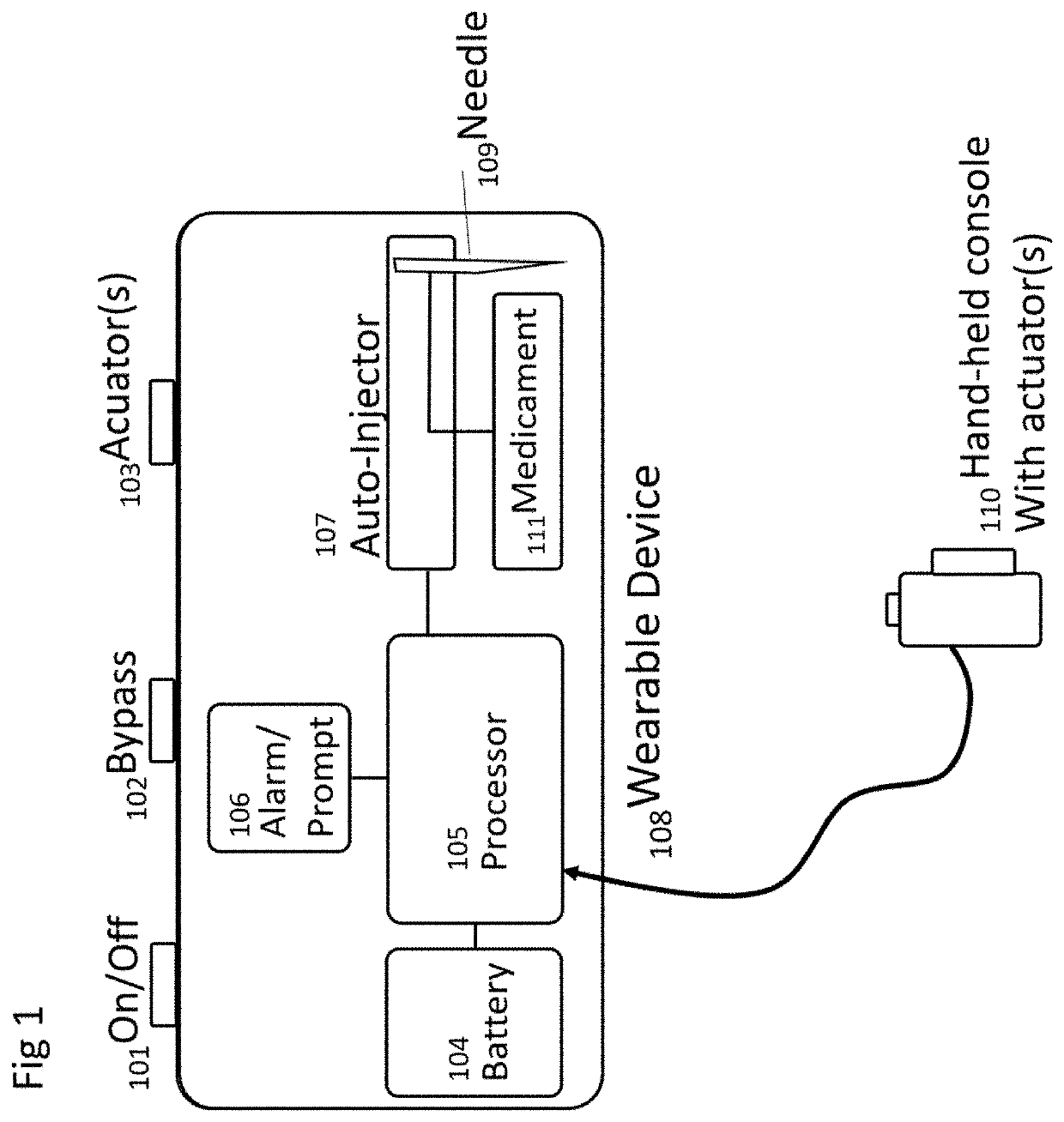
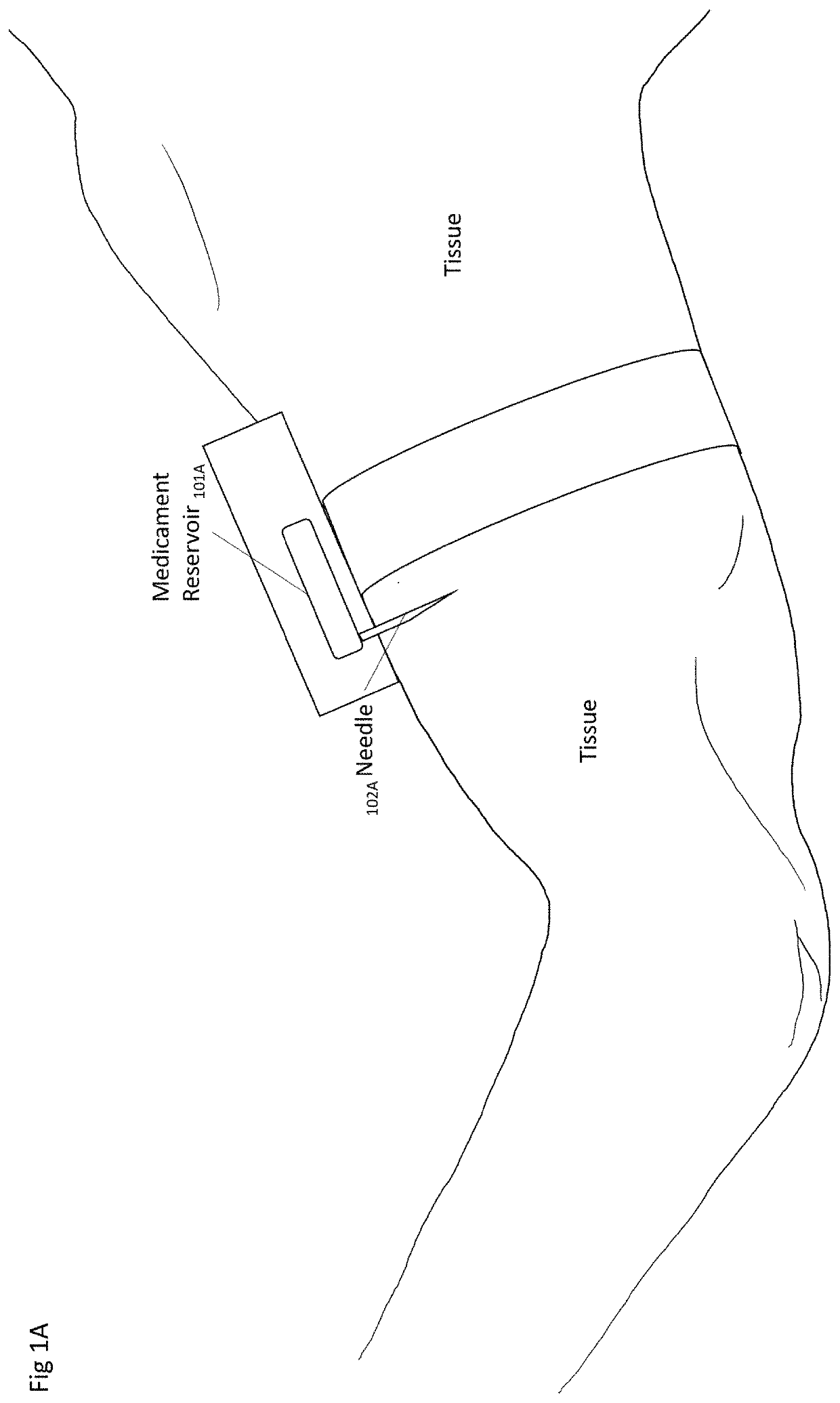
Method and Device for Automatic Identification of an Opioid Overdose and Injection of an Opioid Receptor Antagonist
ActiveUS20170172522A1Constant monitoringRespond effectivelyOrganic active ingredientsAutomatic syringesOpioidergicOpioid overdose
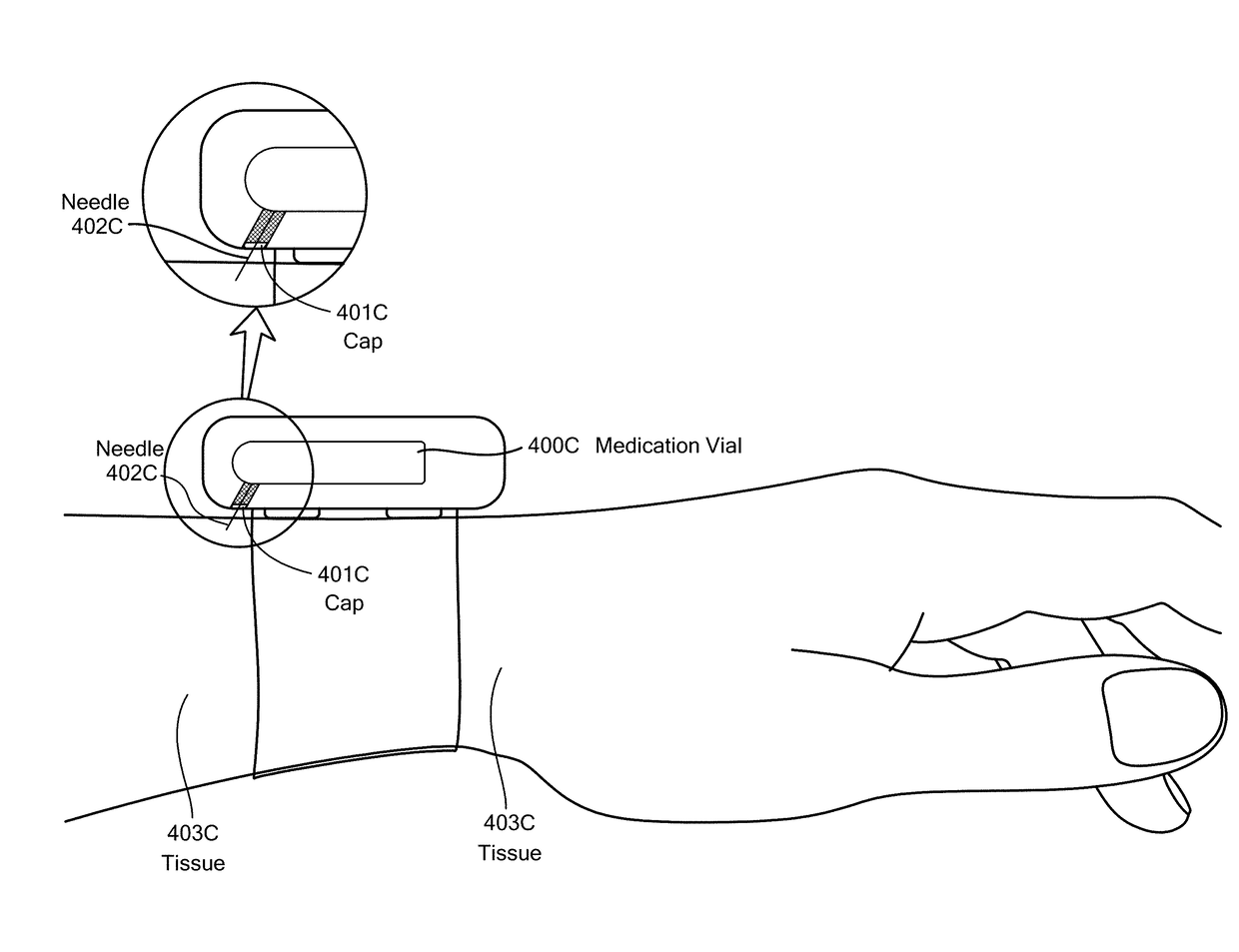
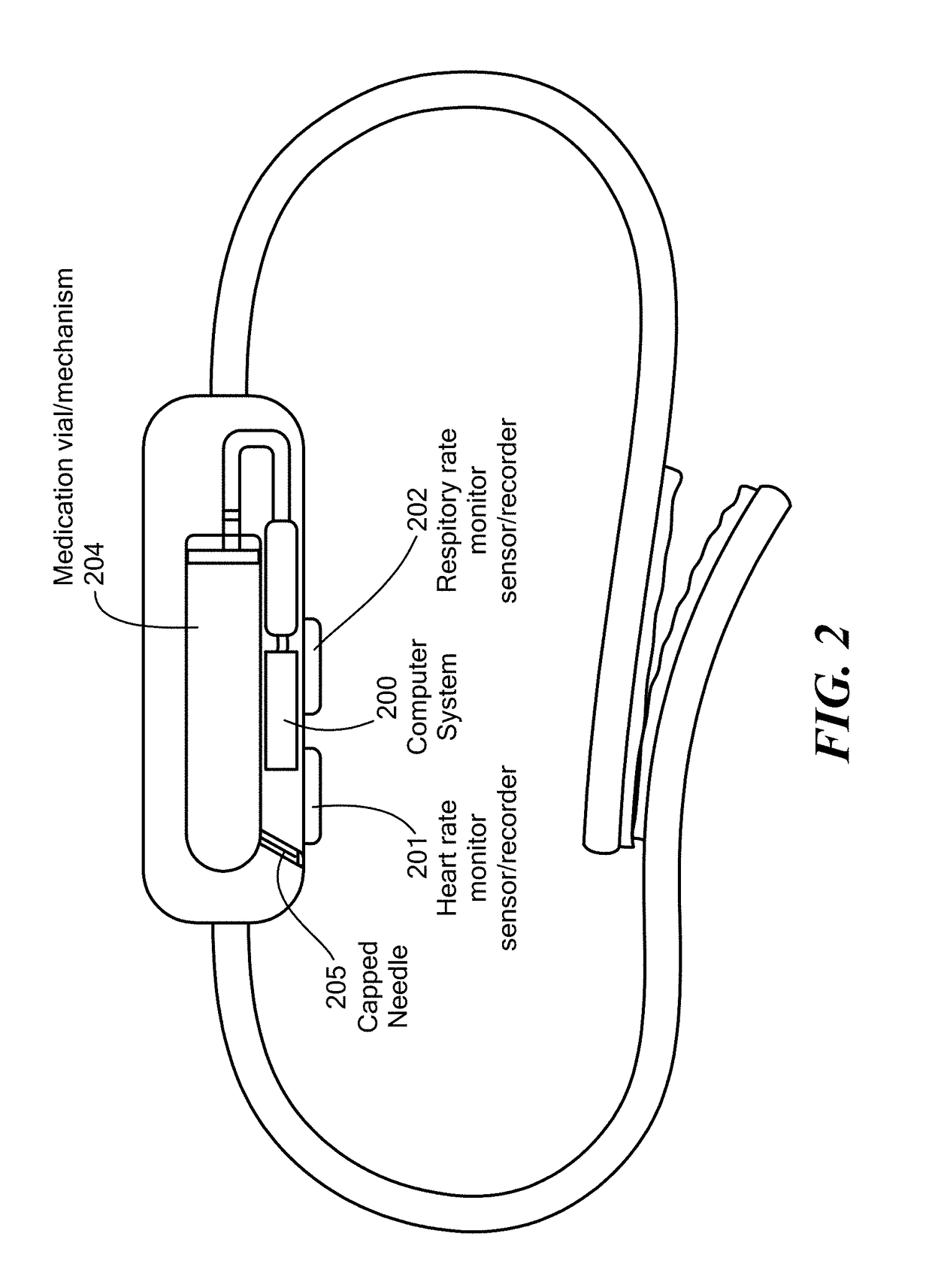
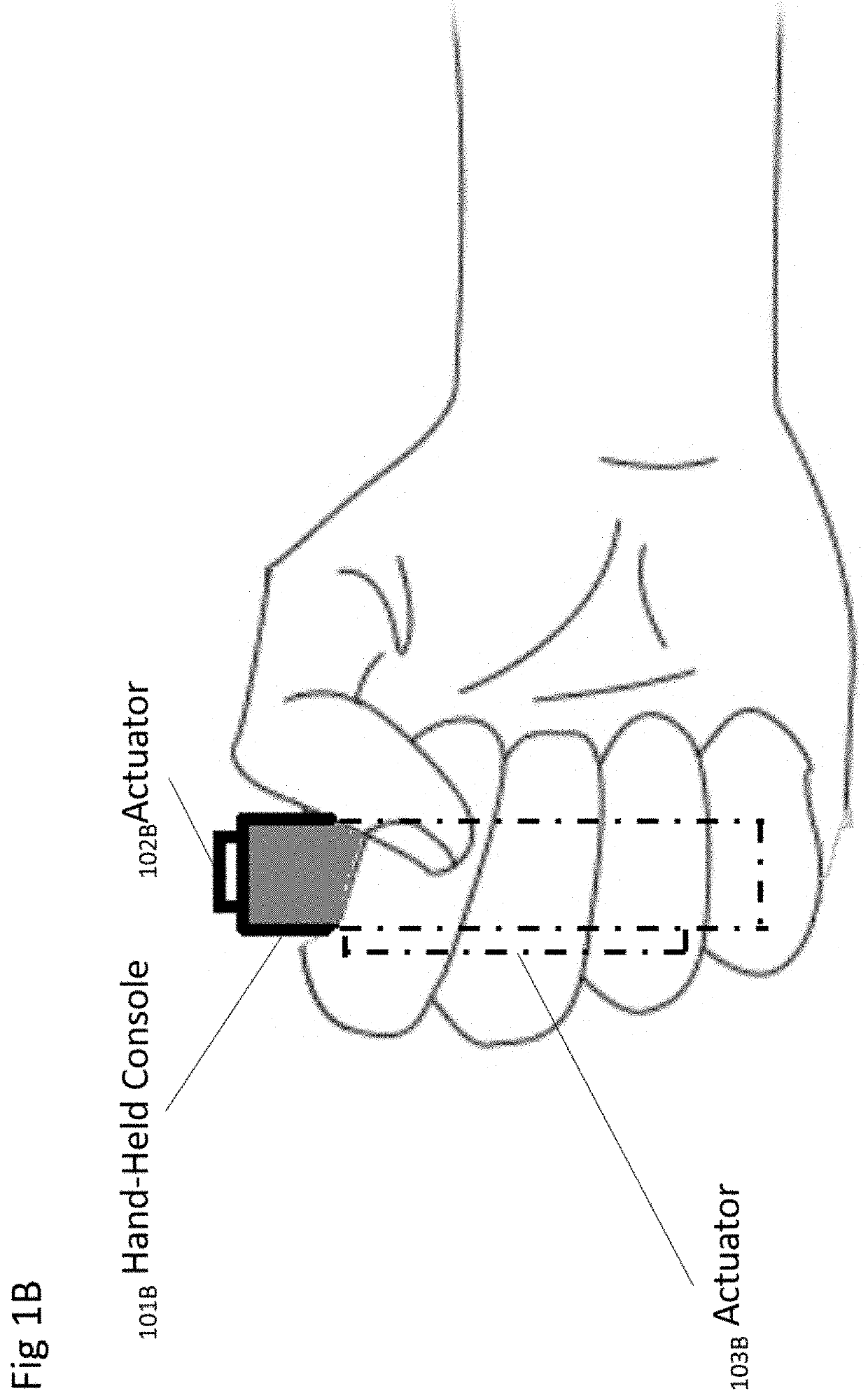
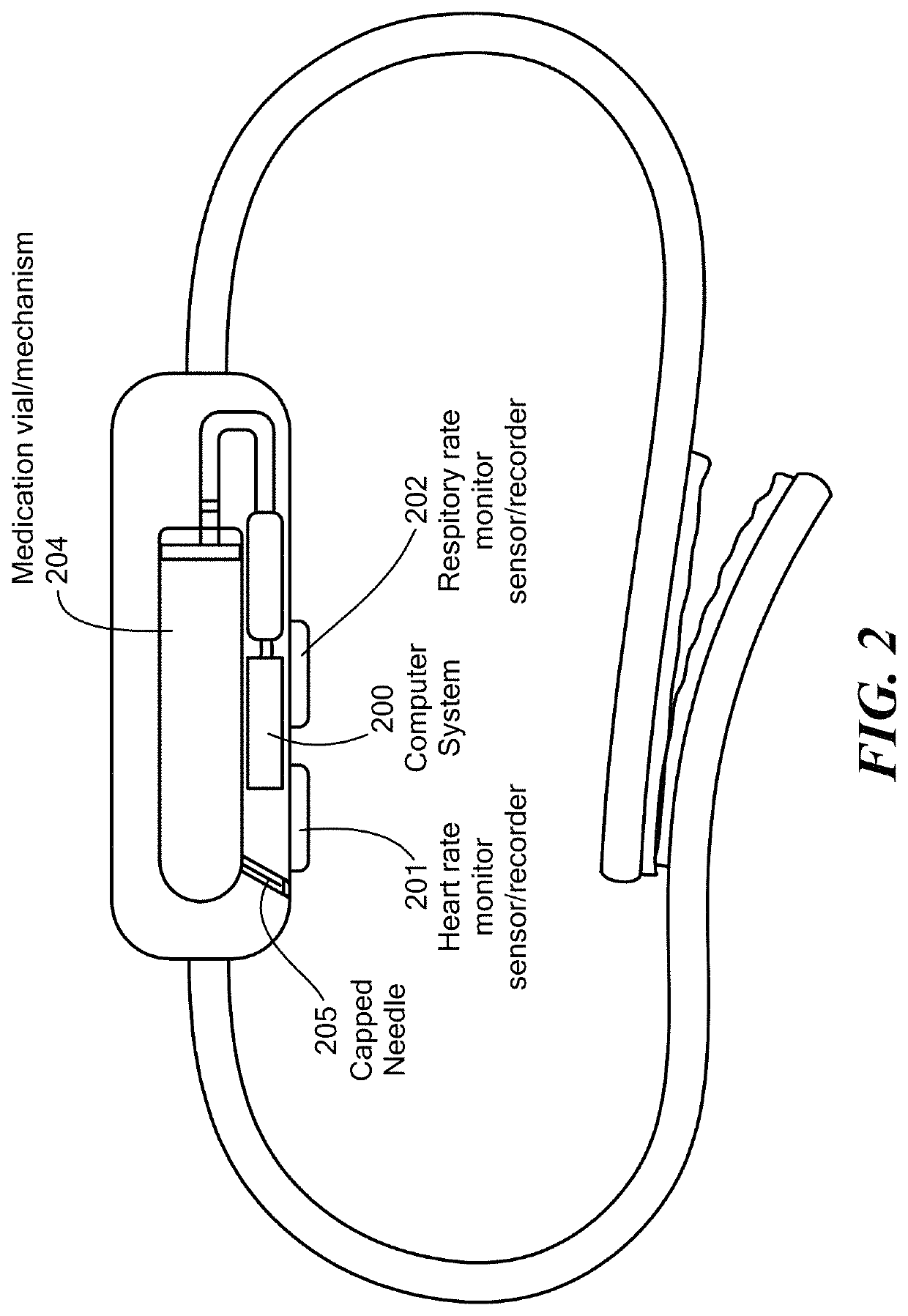
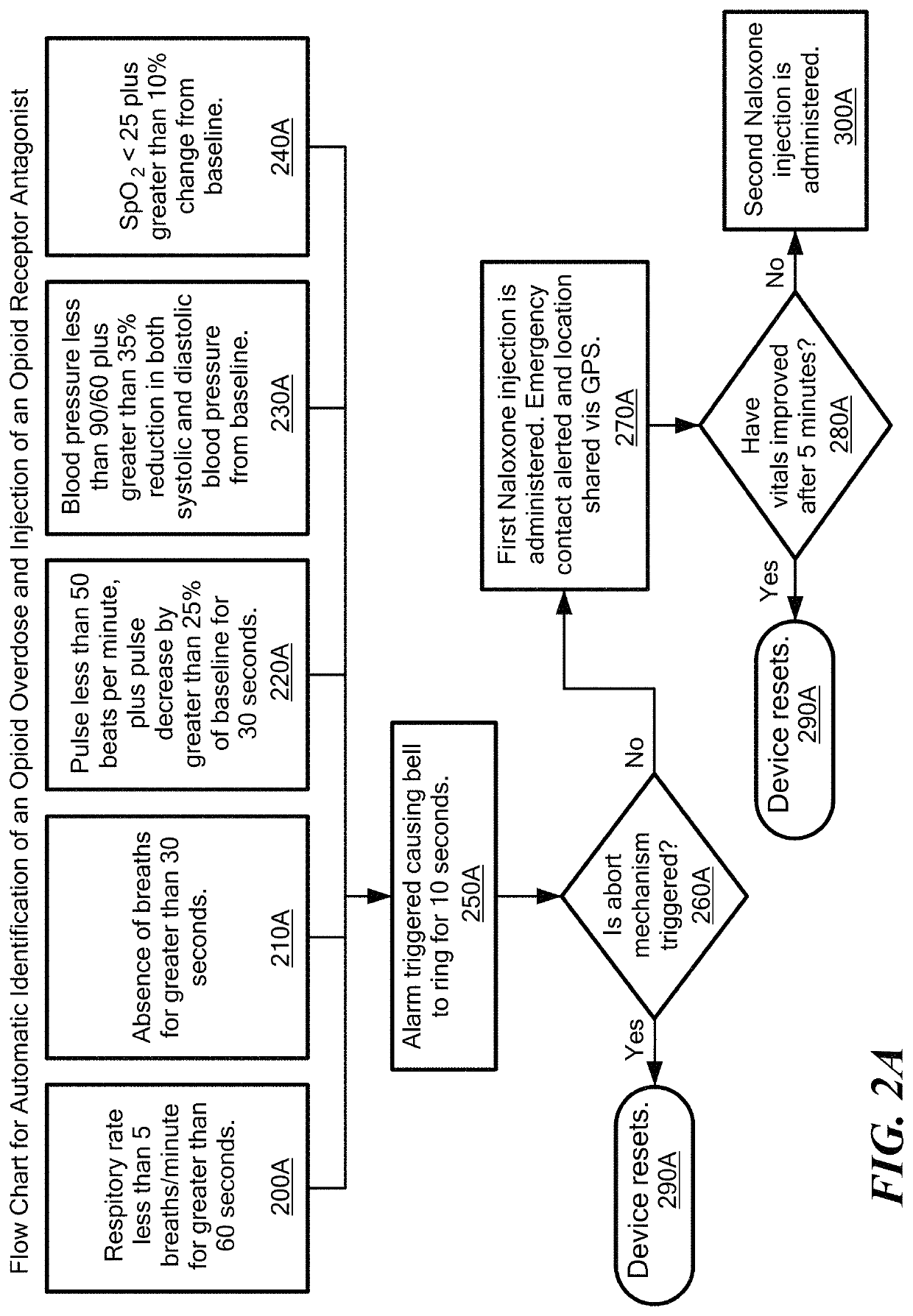
A method for detecting the need for providing assistance to an individual suspected of overdosing on an opiate is provided. The method includes using a wearable device for continuous or intermittent monitoring of one or more physiological parameters of the individual. If the level of one of the one or more physiological parameters exceeds a threshold level specific to that parameter, an alarm is triggered. If the alarm is not aborted, an alert is transmitted to one or more emergency contacts conveying that the individual has overdosed. The method optionally provides for injection of an opioid receptor antagonist into the individual to reverse the effects of the overdose. Also provided is a device for implementing these methods.
Owner:INSLER JOSEPH +1
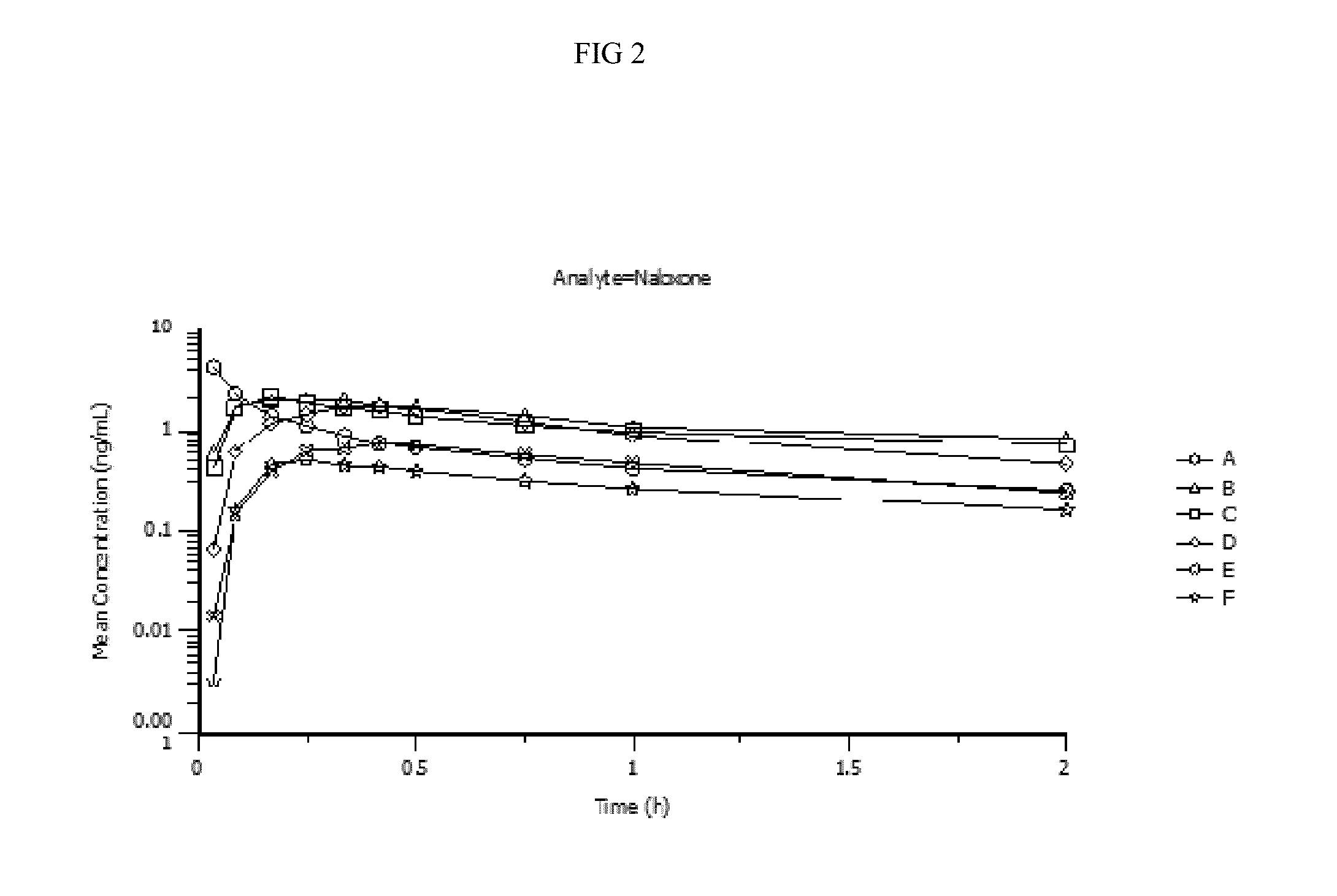
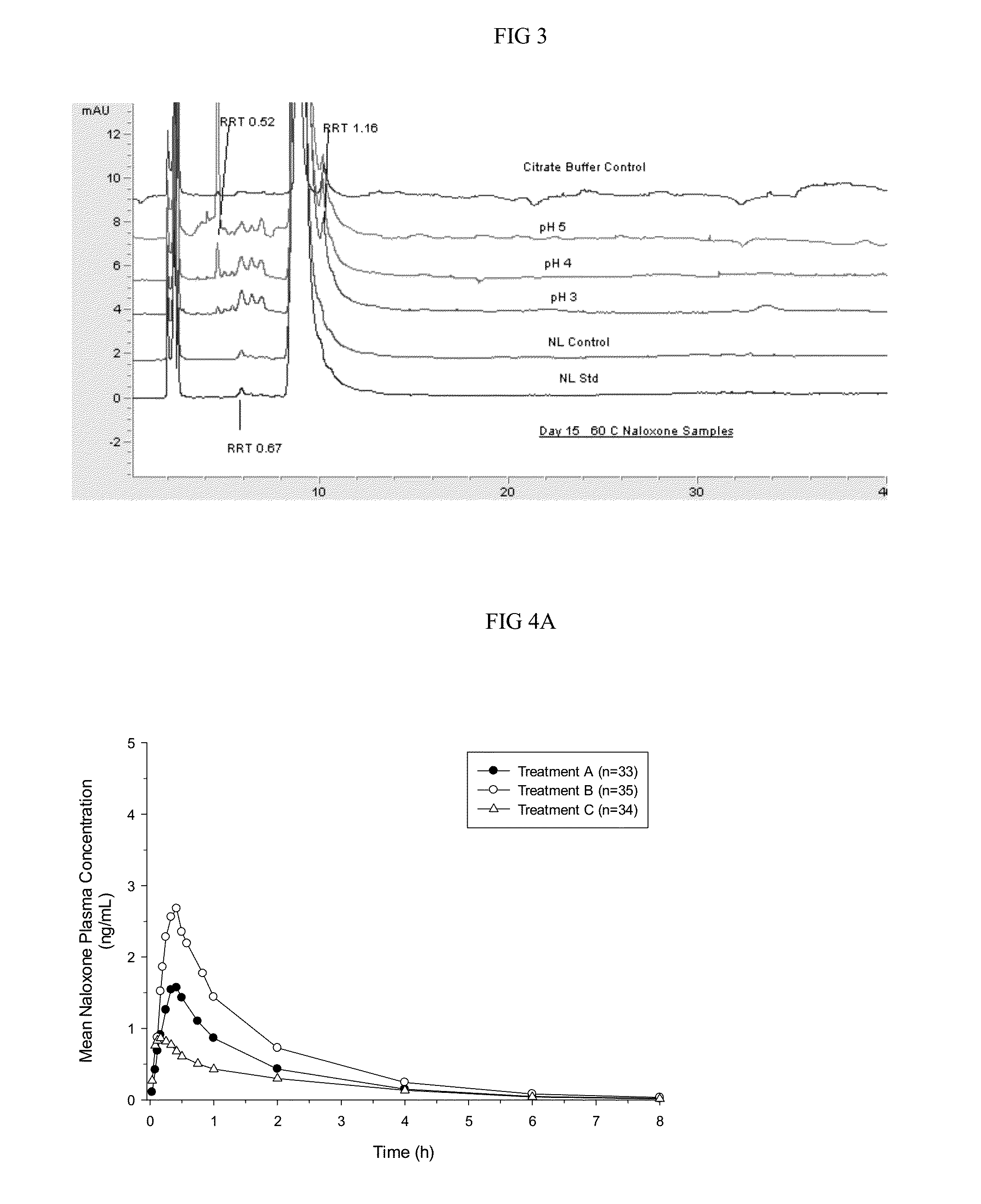
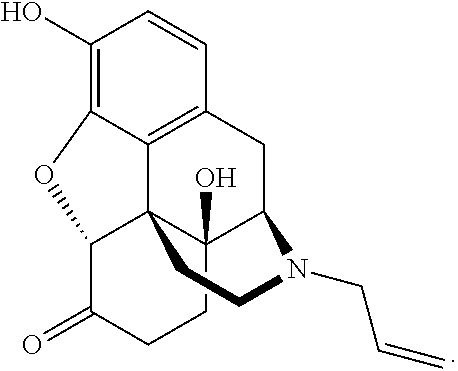
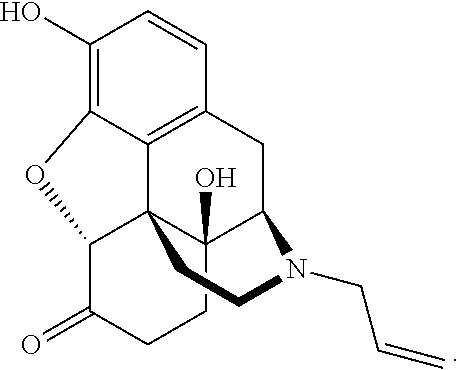
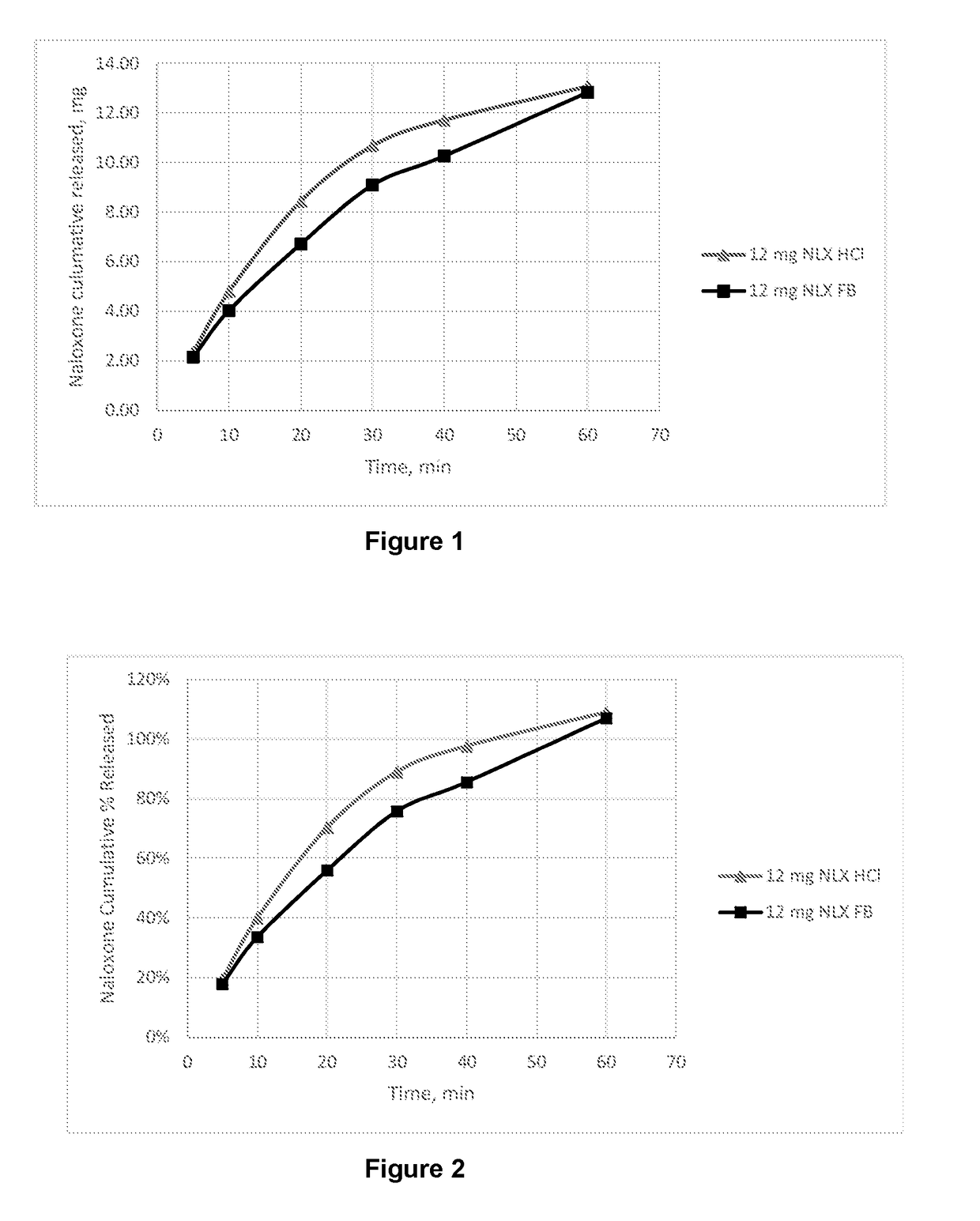
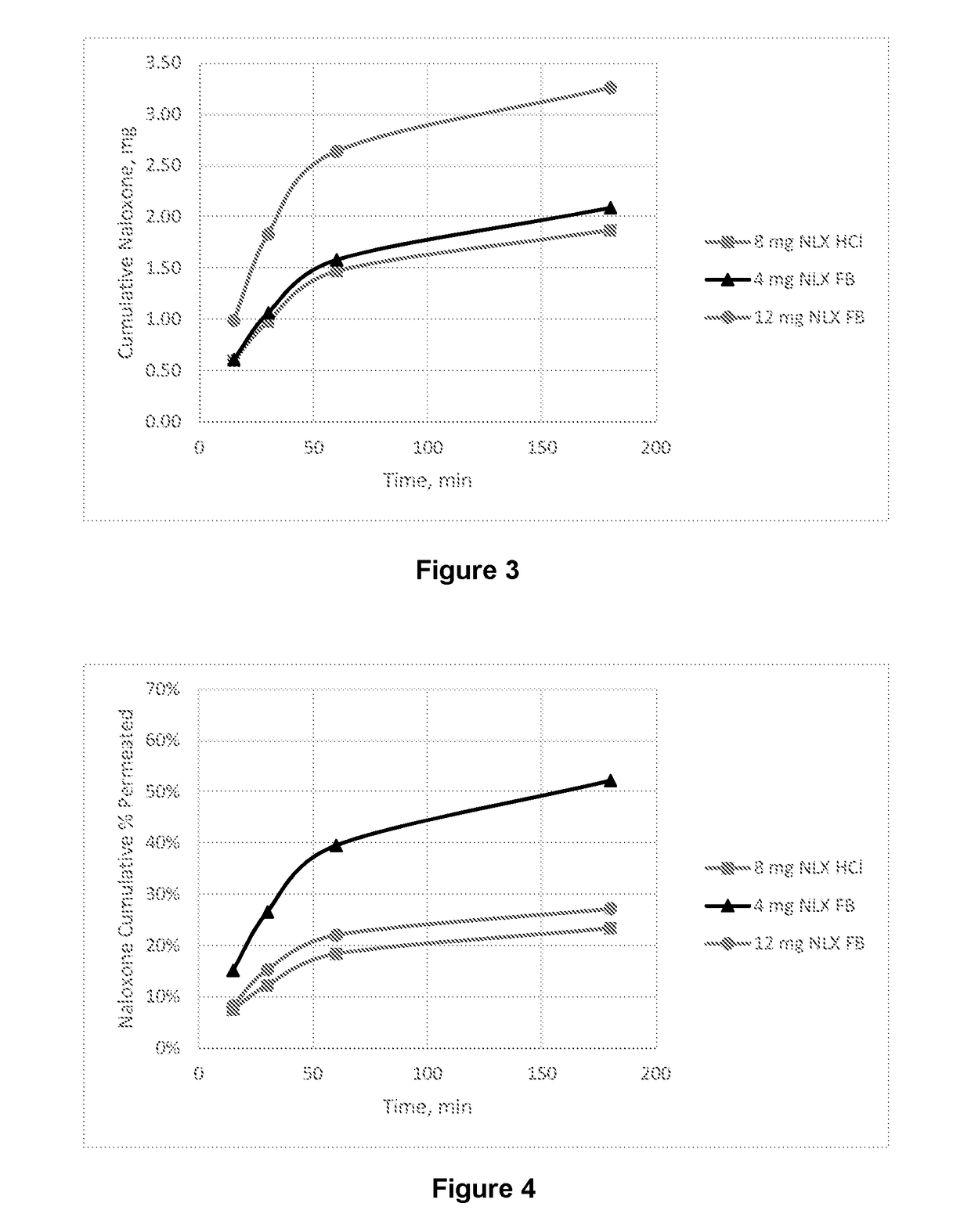
Intranasal naloxone compositions and methods of making and using same
Disclosed herein are compositions containing an opioid antagonist such as naloxone and one or more pharmaceutically acceptable excipients. The compositions may be used for intranasal delivery of Naloxone for the treatment of, for example, opioid overdose in an individual in need thereof. Also disclosed are methods of making compositions containing Naloxone, and devices for nasal delivery of naloxone compositions.
Owner:INDIVIOR UK
Automatic opioid antagonist injection system
InactiveUS20180200433A1Easy injectionOrganic active ingredientsElectrocardiographyOpioid antagonistOpioid overdose
Owner:CIRIT DENIS BARAN
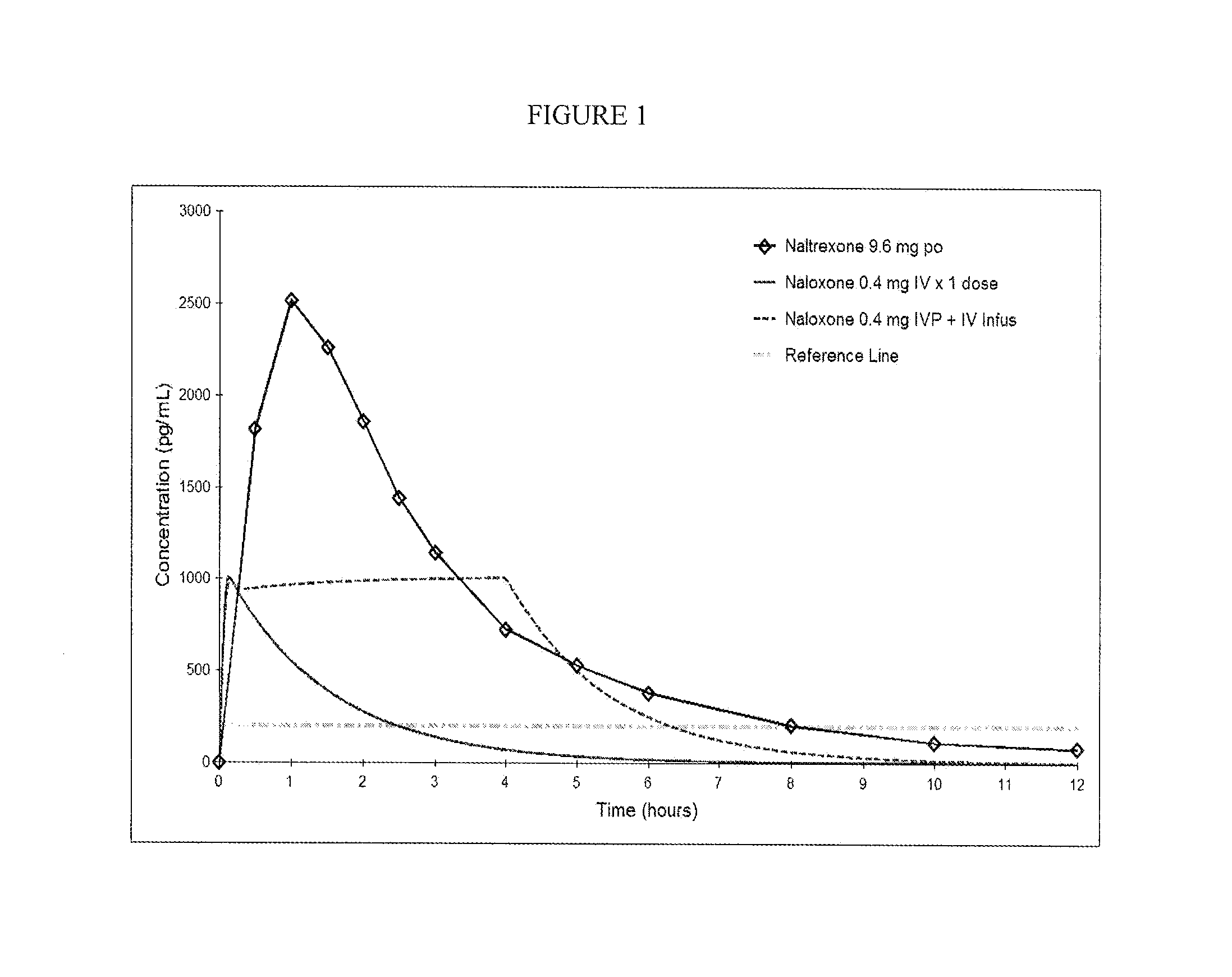
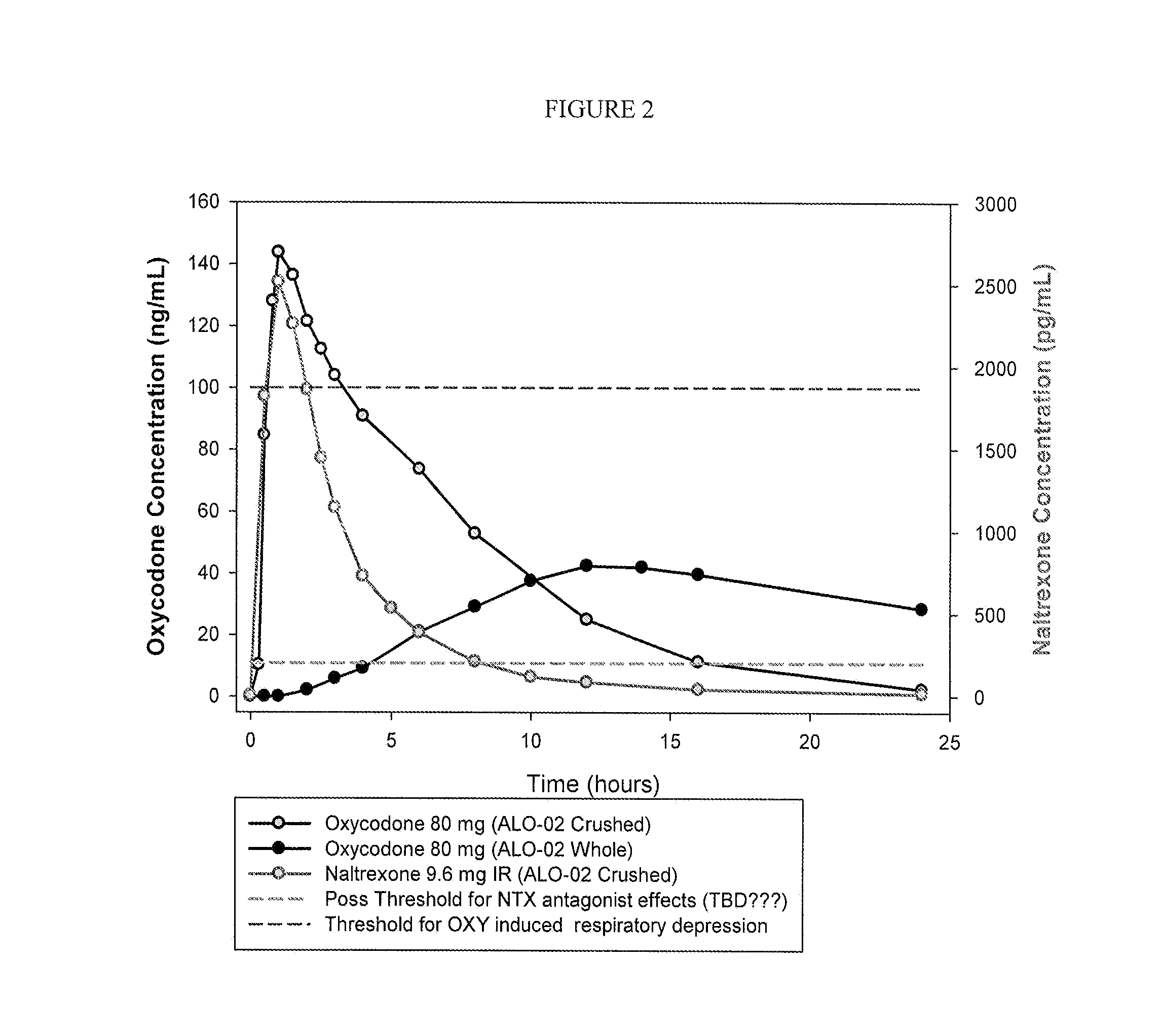
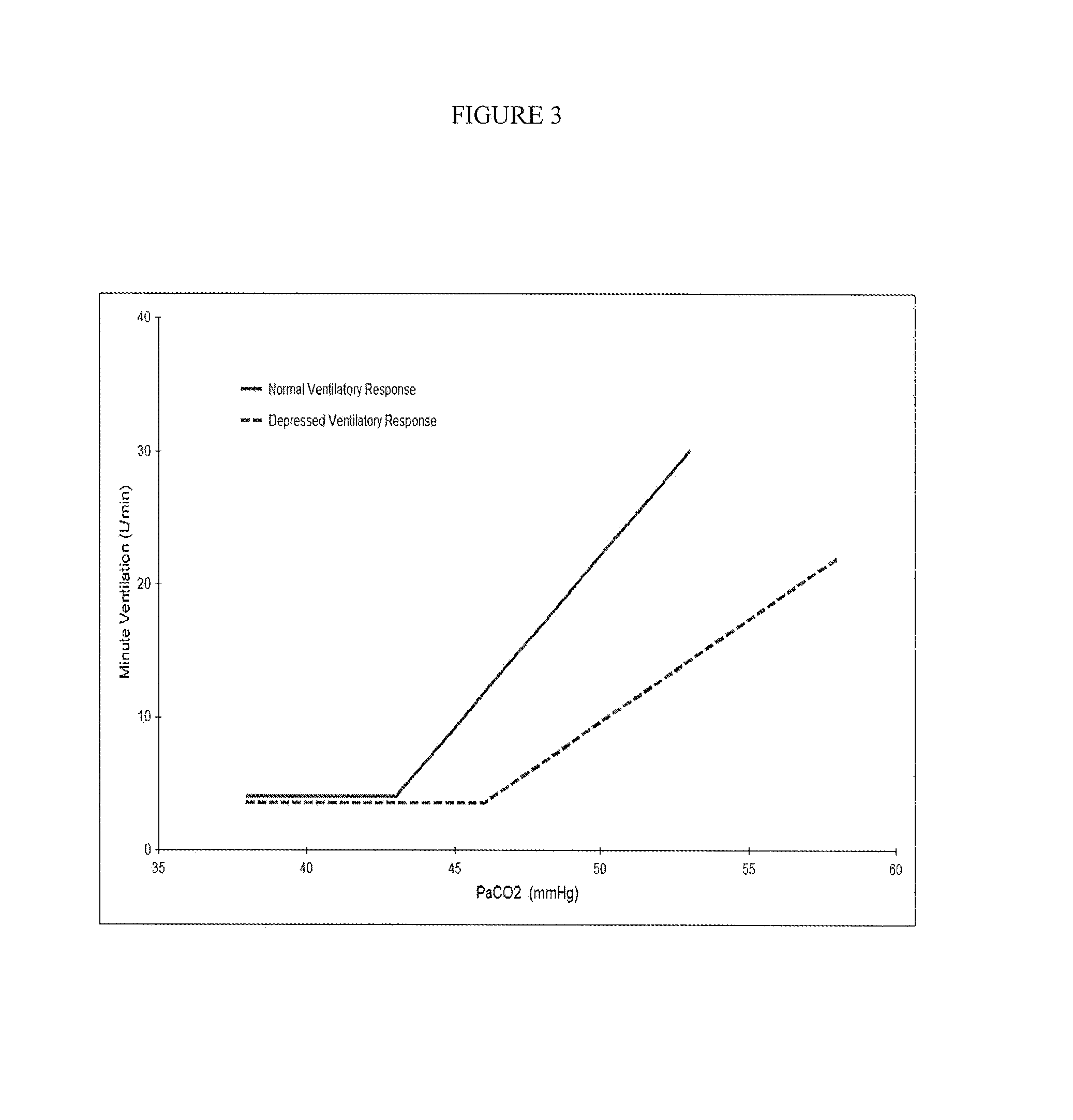
Formulations and Methods for Attenuating Respiratory Depression Induced by Opioid Overdose
Owner:ALPHARMA PHARMA
Intranasal naloxone compositions and methods of making and using same
Disclosed herein are compositions containing an opioid antagonist such as naloxone and one or more pharmaceutically acceptable excipients. The compositions may be used for intranasal delivery of Naloxone for the treatment of, for example, opioid overdose in an individual in need thereof. Also disclosed are methods of making compositions containing Naloxone, and devices for nasal delivery of naloxone compositions.
Owner:INDIVIOR UK
Sublingual naloxone spray
The invention provides storage stable sublingual formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the sublingual formulations of the present invention to a patient in need thereof.
Owner:HIKMA PHARMA USA INC
Intranasal Pharmaceutical Dosage Forms Comprising Naloxone
InactiveUS20150018379A1Improve bioavailabilityQuick effectBiocideNervous disorderMedicineOpioid overdose
The present invention relates to an intranasal pharmaceutical dosage form comprising a dosing unit comprising naloxone or a pharmaceutically acceptable salt thereof in an amount of equivalent to ≧0.5 mg naloxone HCl dissolved in an application fluid of a volume of ≦250 μl. Furthermore, the present invention relates to such an intranasal pharmaceutical dosage form for use in the treatment of opioid overdosing and / or at least one symptom thereof.
Owner:EURO-CELTIQUE SA
Pharmaceutical composition for nasal delivery
ActiveUS10653690B1Improve bioavailabilityFast absorptionOrganic active ingredientsNervous disorderOpioid antagonistOpioidergic
There is provided a solid pharmaceutical composition formulation for nasal delivery of an opioid antagonist. The pharmaceutical composition includes a pharmacologically-effective amount of an opioid antagonist and a pharmaceutically-acceptable carrier. The pharmaceutical composition is preferably in the form of a powder produced by spray-drying, which is subsequently loaded into single use nasal applicators. Preferred pharmaceutically-acceptable carriers include disaccharides (e.g. lactose or trehalose) and dextrins (e.g. cyclodextrins or maltodextrins), preferably spray-dried together in combination. The pharmaceutical composition may further comprise an alkyl saccharide, preferably a sucrose ester, such as sucrose monolaurate. The compositions and applicators may be employed in the treatment of opioid overdose in subjects.
Owner:OREXO AB
Liquid naloxone spray
ActiveUS20190070105A1Improved pharmacokinetic parameterOrganic active ingredientsNervous disorderOpioid overdoseCongenital insensitivity to pain with anhidrosis
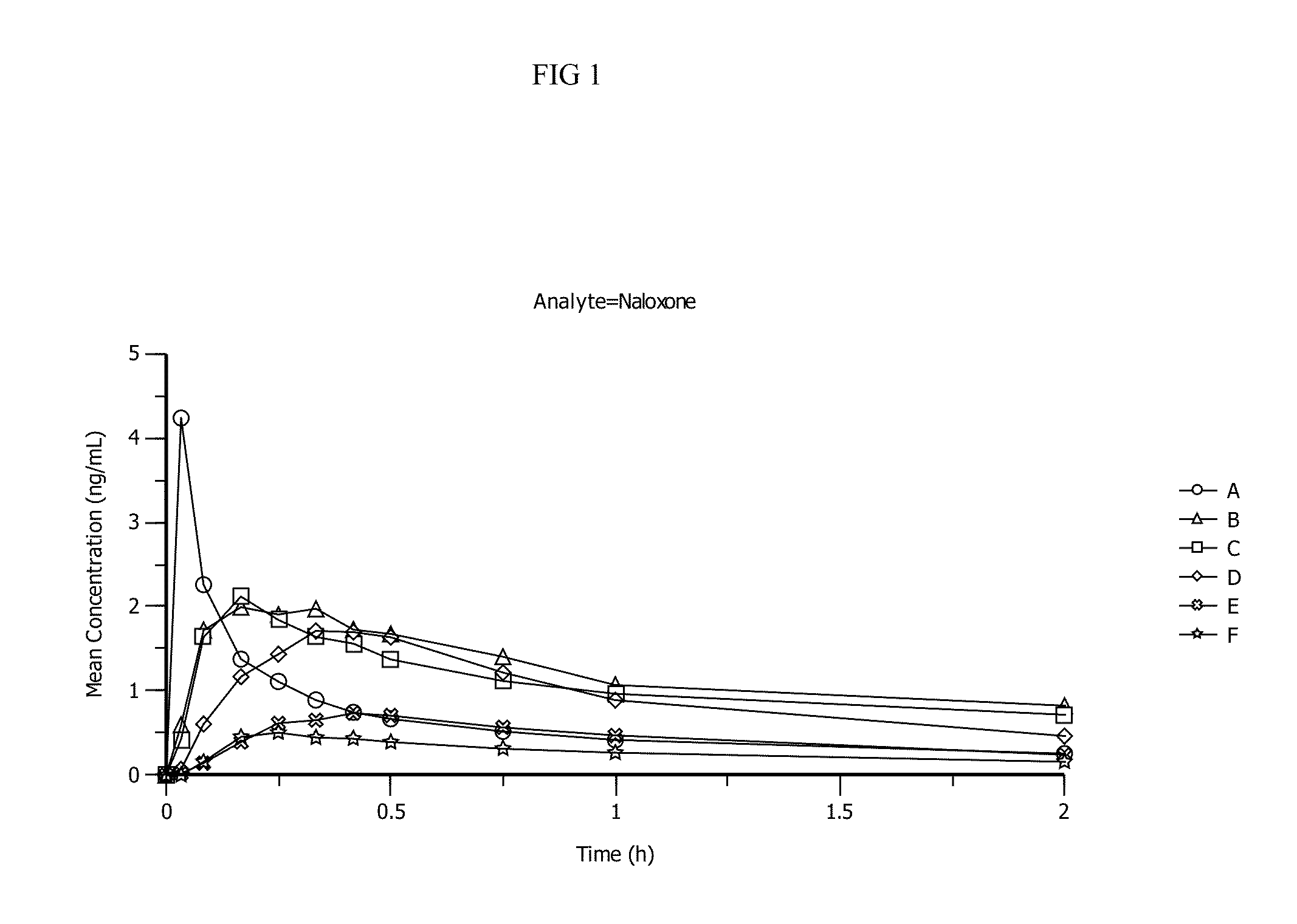
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid overdose, opioid dependence, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Liquid naloxone spray
ActiveUS10441538B2Organic active ingredientsNervous disorderOpioid overdoseCongenital insensitivity to pain with anhidrosis
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid overdose, opioid dependence, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Liquid naloxone spray
ActiveUS10617686B2Organic active ingredientsPharmaceutical delivery mechanismSubstance dependenceOpioid overdose
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
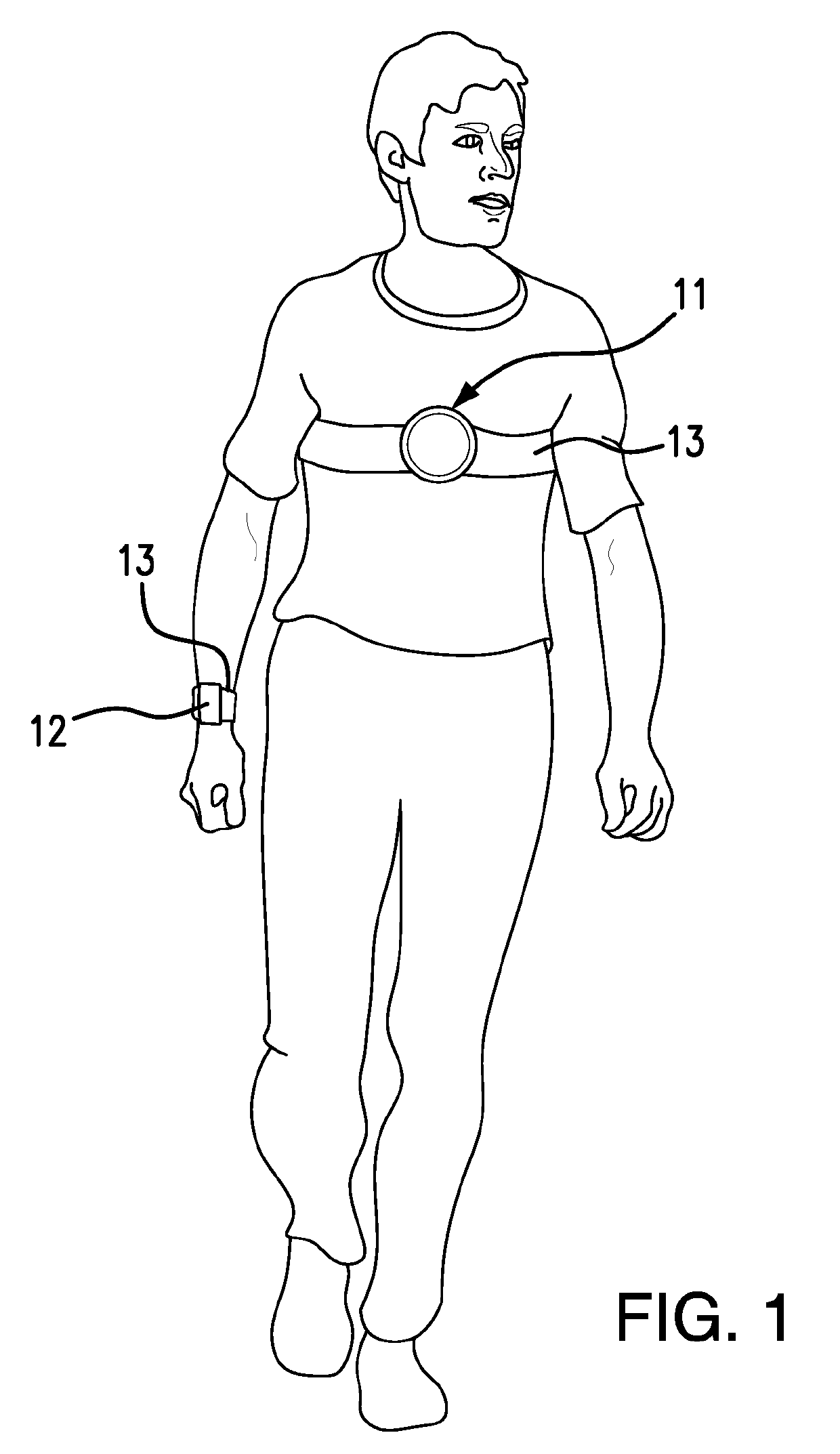
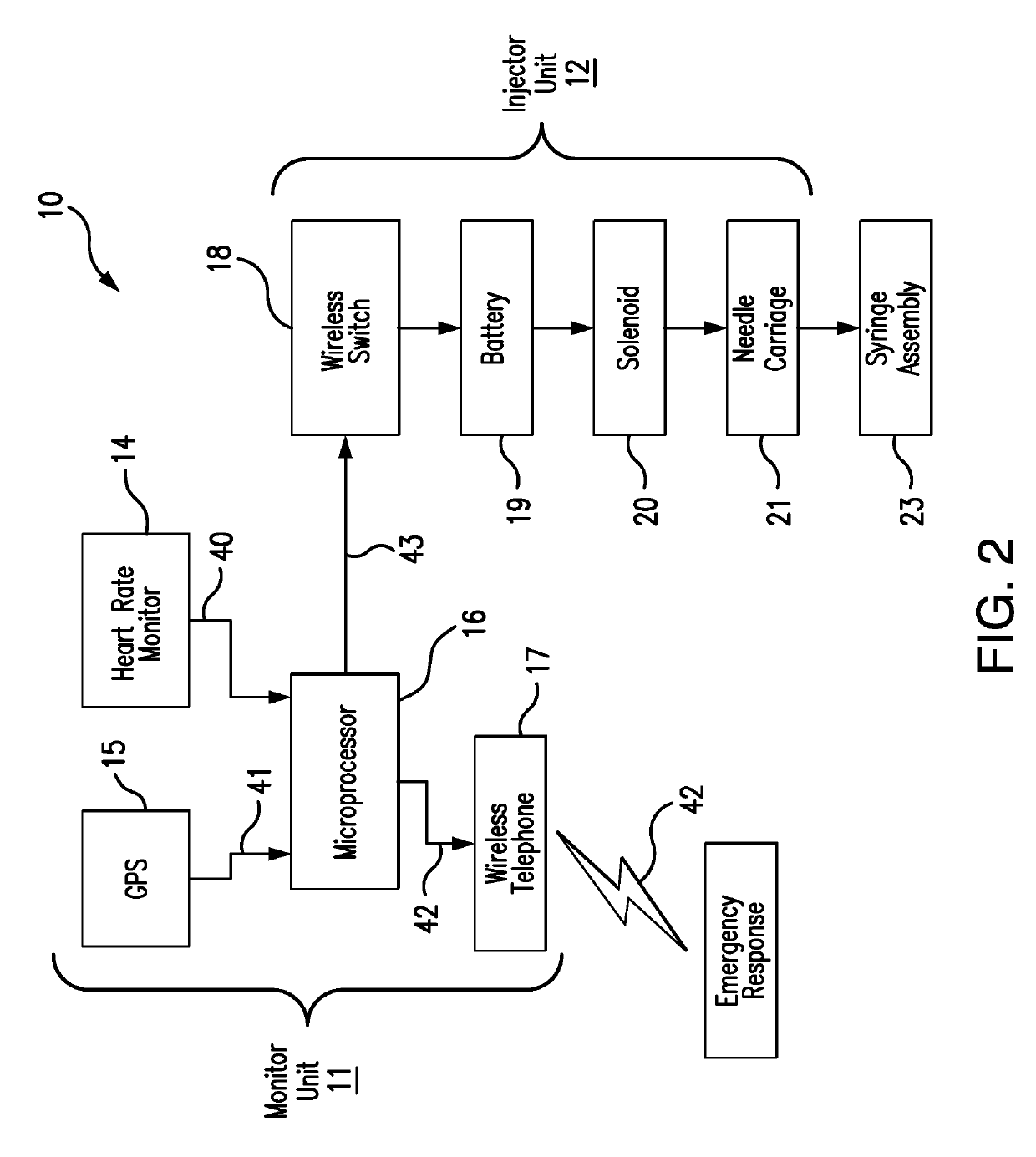
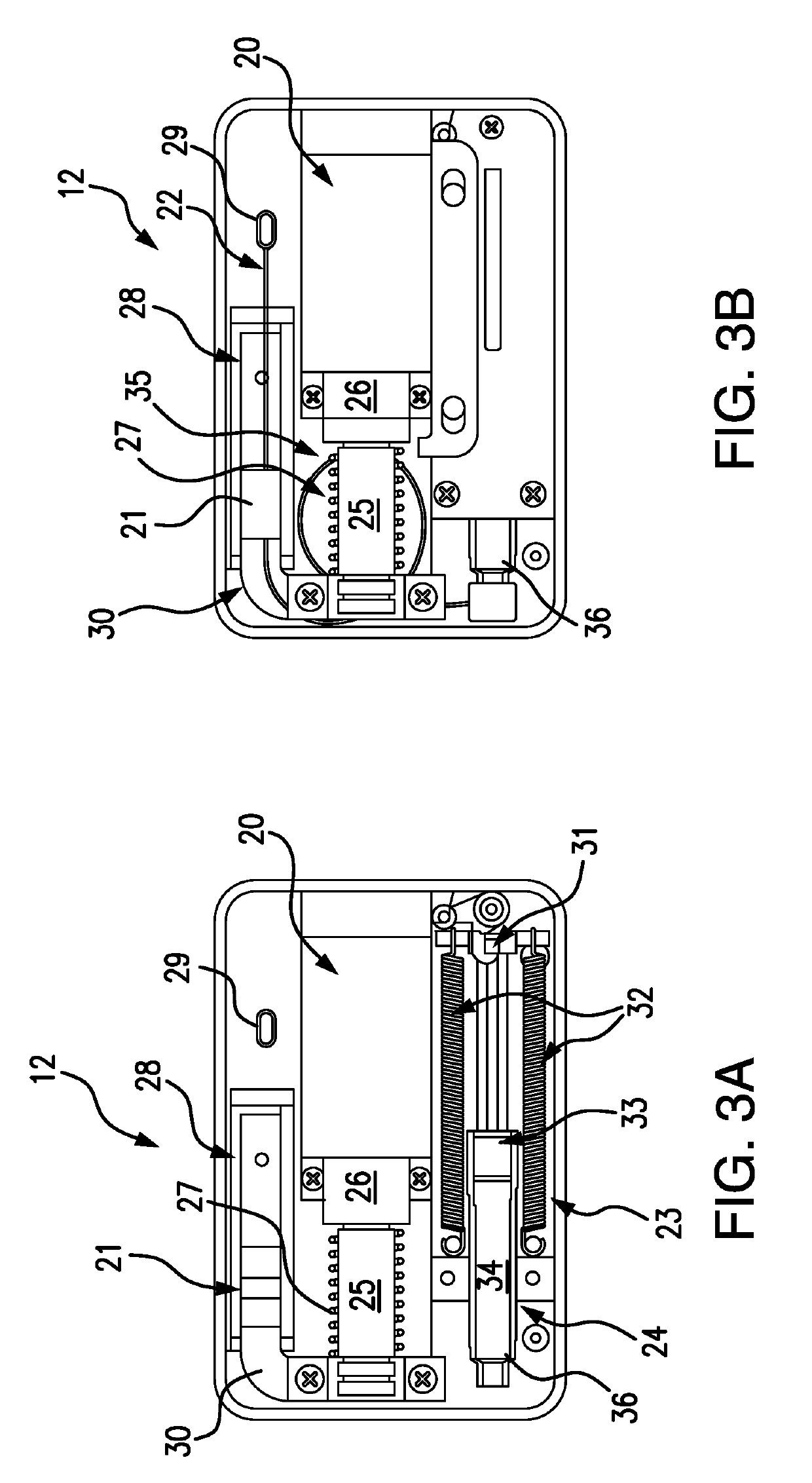
Detection and Response System for Opioid Overdoses
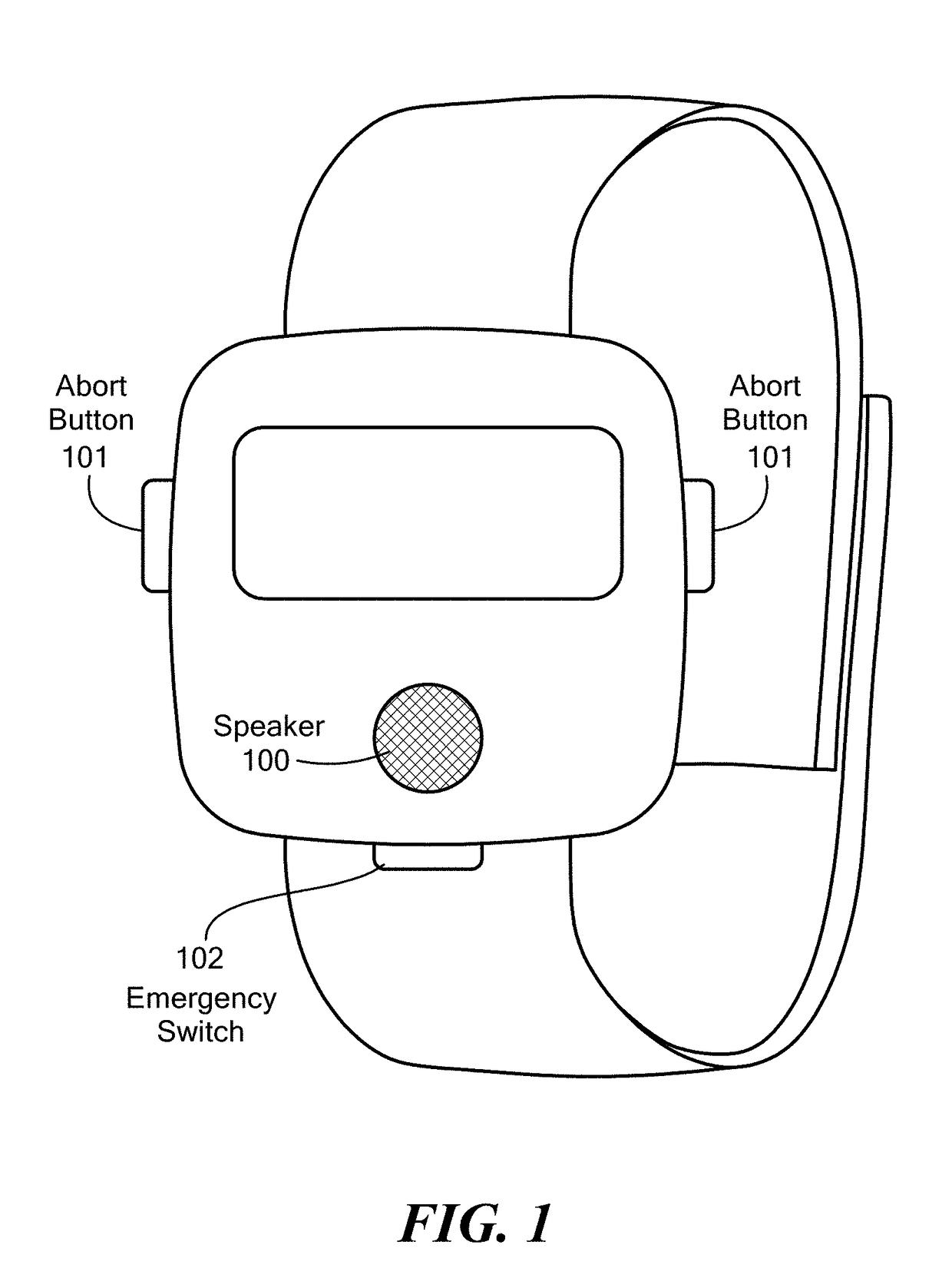
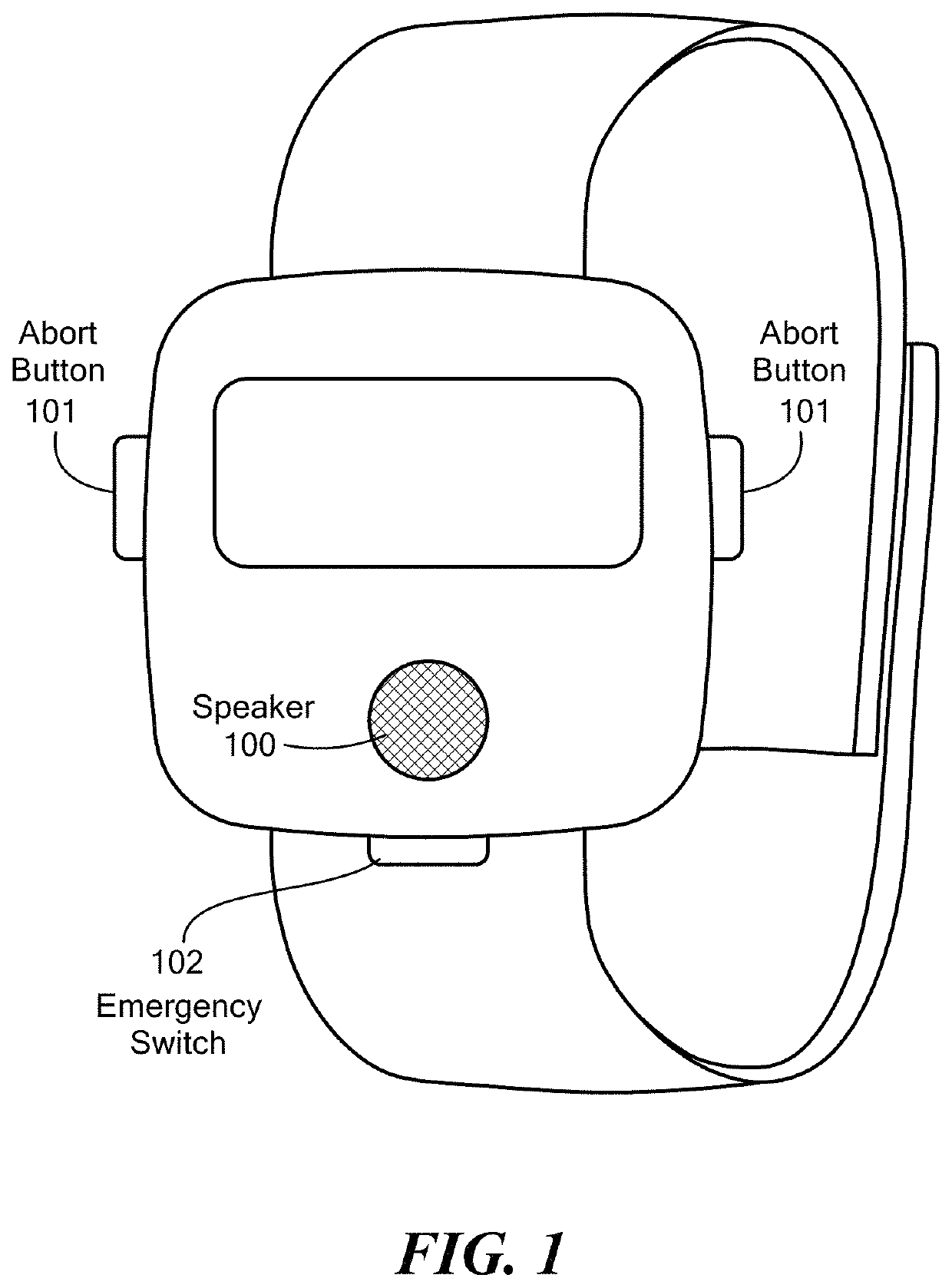
A wearable system detects an opioid overdose and transmits a distress message with the wearer's GPS coordinates to one or more emergency response contacts. Concurrently, the system signals a switch which energizes a solenoid injector, causing a prescribed dosage of an opioid antidote to be injected by a syringe into the wearer's body. Detection of an opioid overdose is based on one or more symptomatic biometrics, which are measured by a wearable monitor. The monitor unit and the injector units can be separate, or they can be combined in a single unit.
Owner:GRANDE VINCENZO
Sublingual naloxone spray
ActiveUS20160008349A1BiocideNervous disorderOpioid overdoseCongenital insensitivity to pain with anhidrosis
The invention provides storage stable sublingual formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the sublingual formulations of the present invention to a patient in need thereof.
Owner:HIKMA PHARMA USA INC
Sublingual naloxone spray
InactiveUS20160199294A1BiocidePharmaceutical delivery mechanismOpioid overdoseCongenital insensitivity to pain with anhidrosis
The invention provides storage stable sublingual formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the sublingual formulations of the present invention to a patient in need thereof.
Owner:HIKMA PHARMA USA INC
Liquid naloxone spray
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid overdose, opioid dependence, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Liquid naloxone spray
ActiveUS10973814B2Organic active ingredientsPharmaceutical delivery mechanismSubstance dependenceOpioid overdose
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid overdose, opioid dependence, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Liquid naloxone spray
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid overdose, opioid dependence, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Liquid naloxone spray
ActiveUS10722510B2Organic active ingredientsInorganic non-active ingredientsSubstance dependenceOpioid overdose
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention both intranasally and sublingually to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally and sublingually the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Apparatus and methods for rapid transmucosal drug delivery
InactiveUS20190070396A1Organic active ingredientsMedical devicesOpioid overdoseBULK ACTIVE INGREDIENT
Medical devices and methods for rapid and efficient systemic drug delivery via mucous membranes, particularly oral mucosae, are described. Use of such devices and methods are particularly easy for people without any medical training to employ should they be called upon to provide emergency medical treatment for a victim suffering from anaphylactic shock, opioid overdose, or other life-threatening events. In some embodiments, the device includes an applicator tip with a porous application layer positioned on the end of an elongate handle. The device may also include various means for disrupting a barrier to facilitate mixing of compounds, including an active ingredient, and permit flow of the compounds from a reservoir to the applicator tip.
Owner:STATIM PHARMA INC
Formulations and Methods for Attenuating Respiratory Depression Induced by Opioid Overdose
Owner:ALPHARMA PHARMA
Liquid naloxone spray
ActiveUS20160354363A1Organic active ingredientsInorganic non-active ingredientsOpioid overdosePharmacology
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention both intranasally and sublingually to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally and sublingually the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Pharmaceutical composition for nasal delivery
ActiveUS10729687B1Improve bioavailabilityFast absorptionPowder deliveryOrganic active ingredientsOpioid antagonistOpioidergic
According to the invention, there is provided a solid pharmaceutical composition formulation for nasal delivery of an opioid antagonist, comprising a pharmacologically-effective amount of an opioid antagonist and a pharmaceutically-acceptable carrier. The compositions are preferably in the form of a powder produced by spray-drying, which are subsequently loaded into single use nasal applicators. Preferred pharmaceutically-acceptable carriers in this regard include disaccharides (e.g. lactose or trehalose) and dextrins (e.g. cyclodextrins or maltodextrins), preferably spray-dried together in combination. Compositions may further comprise an alkyl saccharide, preferably a sucrose ester, such as sucrose monolaurate. The compositions and applicators may be employed in the treatment of opioid overdose in subjects.
Owner:OREXO AB
Liquid naloxone spray
ActiveUS20170252337A1Organic active ingredientsPharmaceutical delivery mechanismOpioid overdoseCongenital insensitivity to pain with anhidrosis
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Liquid naloxone spray
InactiveCN109922805AOrganic active ingredientsPharmaceutical delivery mechanismOpioidergicOpioid overdose
The invention provides stable liquid formulations containing naloxone, a pharmaceutically acceptable salt, or a derivative thereof. The invention further provides methods for treating opioid dependence, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering the liquid formulations of the present invention intranasally to a patient in need thereof. Further, the invention provides a method of treating opioid dependence-, opioid overdose, and congenital insensitivity to pain with anhidrosis by administering intranasally the naloxone formulations of the present invention.
Owner:HIKMA PHARMA USA INC
Pharmaceutical formulation
ActiveUS20200246253A1Reduce riskDesirable balance of propertyOrganic active ingredientsNervous disorderOpioidergicOpioid overdose
The present invention relates to a film comprising an alginate salt of a monovalent cation or a mixture of alginate salts containing at least one alginate salt of a monovalent cation, and an antagonist of an opioid receptor, an inverse agonist of an opioid receptor, or a prodrug thereof. The present invention further relates to methods for manufacturing such a film, and the use of such a film in the treatment of a human patient, in particular the use of such a film in the treatment of the effects of acute opioid overdose, or the use of such a film in reducing the risk of opioid abuse.
Owner:KLARIA PHARMA HLDG AB
Formulations and Methods for Attenuating Respiratory Depression Induced by Opioid Overdose
Owner:ALPHARMA PHARMA
Pharmaceutical composition for nasal delivery
According to the invention, there is provided a solid pharmaceutical composition formulation for nasal delivery of an opioid antagonist, comprising a pharmacologically-effective amount of an opioid antagonist and a pharmaceutically-acceptable carrier. The compositions are preferably in the form of a powder produced by spray-drying, which are subsequently loaded into single use nasal applicators. Preferred pharmaceutically-acceptable carriers in this regard include disaccharides (e.g. lactose or trehalose) and dextrins (e.g. cyclodextrins or maltodextrins), preferably spray-dried together in combination. Compositions may further comprise an alkyl saccharide, preferably a sucrose ester, such as sucrose monolaurate. The compositions and applicators may be employed in the treatment of opioid overdose in subjects.
Owner:OREXO AB
Dosage form for administration of opioid antagonists
InactiveUS20180228728A1Reduce and counteract effectOrganic active ingredientsNervous disorderOpioid antagonistOpioid Agonist
A novel dosage form for delivering an opioid antagonist such as naloxone is disclosed. The dosage form comprises an oral dissolvable film containing an amount of the opioid antagonist effective to reduce or counteract the effect of an opioid overdose in an individual. Methods of administering the dosage form to an individual experiencing an opioid overdose are also disclosed.
Owner:CHARTWELL TRANSDERMALS LLC
Systems and method for detection and treatment of opioid overdose
InactiveUS20200147307A1Low costAlleviates regulatory requirementOrganic active ingredientsOrganic chemistryOpioidergicPhysical medicine and rehabilitation
A device and method utilizing user operated actuators such as momentary switches, intermediate switches, accelerometers or video cameras to detect impaired consciousness as an early symptom of opioid overdose to then trigger administration of preloaded naloxone to rescue the user from an overdose.
Owner:YAP STEVEN DR
Method and device for automatic identification of an opioid overdose and injection of an opioid receptor antagonist
ActiveUS10874358B2Constant monitoringRespond effectivelyOrganic active ingredientsAutomatic syringesOpioidergicOpioid overdose
Owner:INSLER JOSEPH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com