Cyclobenzaprine sustained-release preparation
A technology of cyclobenzaprine and sustained-release tablets, applied in the field of medicine, can solve the problems of affecting the curative effect, slow elimination, poor patient compliance, etc., and achieve the effects of stable product quality, reduced dosage, and uniform drug release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] A preparation method of cyclobenzaprine sustained-release tablet, comprising the following steps:
[0024] (1) get cyclobenzaprine and filler to mix evenly by weight percentage, take 85% ethanol as binding agent, make soft material, 20-24 mesh sieve granulation;
[0025] (2) Add surfactants and lubricants to the granules prepared in step (1), mix well, press into tablets, and dry to obtain cyclobenzaprine sustained-release tablets.
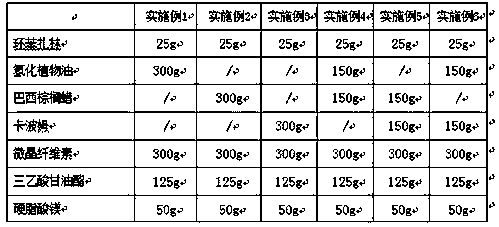
Embodiment 1-6
[0026] The preparation of embodiment 1-6 cyclobenzaprine sustained release tablet
[0027] According to the raw and auxiliary materials in the following prescription, the cyclobenzaprine sustained-release tablet is prepared according to the above-mentioned preparation method. Among them, " / " means not used.
[0028]
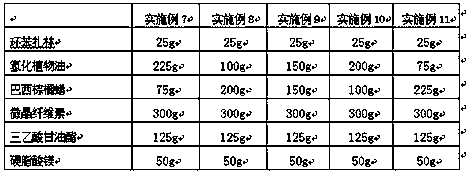
Embodiment 7-11
[0034] Example 7-11 Preparation of Cyclobenzaprine Sustained Release Tablets
[0035] According to the raw and auxiliary materials in the following prescription, the cyclobenzaprine sustained-release tablet is prepared according to the above-mentioned preparation method.
[0036]
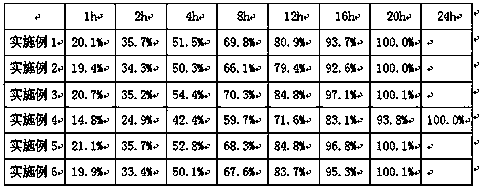
[0037] Test Example 2 The Cyclobenzaprine Sustained-release Tablet Release Determination of Embodiment 7-11 Gained
[0038] The measurement method is the same as that of Test Example 1, and the measurement results are shown in Table 2.
[0039] Table 2 The release investigation table of cyclobenzaprine sustained-release tablets obtained in Examples 7-11
[0040]
[0041] As can be seen from Table 2, the cyclobenzaprine sustained-release tablet of embodiment 9 releases slowly within 24 hours, indicating that when hydrogenated vegetable oil and carnauba wax are used as sustained-release materials, and the weight ratio is 1:1, the prepared Cyclobenzaprine sustained-release tablets have the best...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com