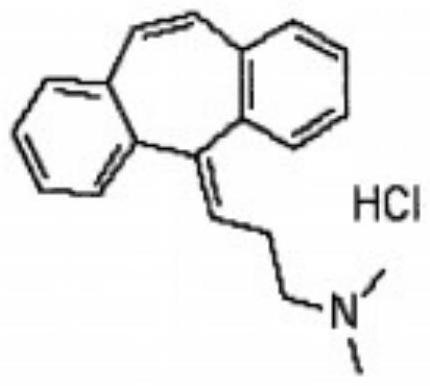
A kind of cyclobenzaprine hydrochloride sublingual tablet and preparation method thereof
A technology of cyclobenzaprine hydrochloride and sublingual tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., to achieve stable quality, enhanced drug experience, and remarkable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
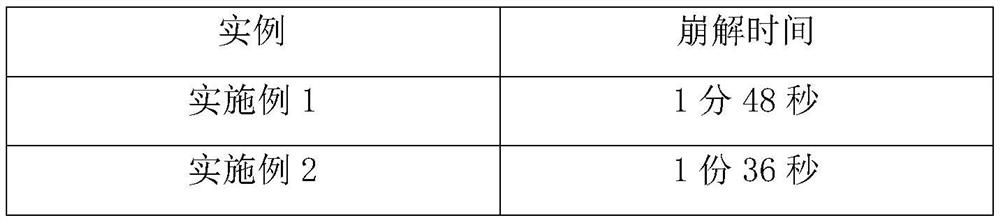
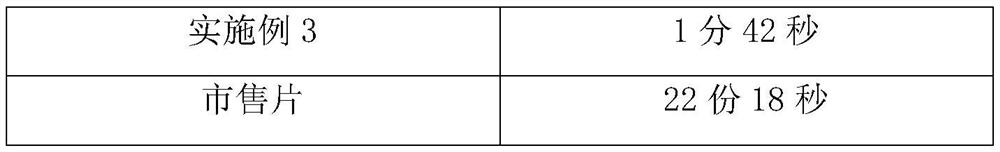
[0025] Weigh each component according to the following ratio: cyclobenzaprine hydrochloride 20%, sucrose-lactose-mannitol 40% with a weight ratio of 3:2:5, microcrystalline cellulose 15%, carboxymethylcellulose calcium C 10%, 3% sodium saccharin, an appropriate amount of orange flavor, an appropriate amount of sorbic acid, and an appropriate amount of distilled water. Weigh the diluent in proportion, mix it evenly, and pass through an 80-mesh sieve; mix the prepared diluent with cyclobenzaprine hydrochloride, disintegrating agent, corrective agent, essence and preservative, add distilled water to granulate, Lubricant is added and compressed into tablets.
Embodiment 2
[0027] Cyclobenzaprine Hydrochloride 25%, Sucrose-Lactose-Mannitol 3:2:5 by weight 40%, Microcrystalline Cellulose 10%, Carmellose Calcium C 10%, Sodium Saccharin 3%, Orange Flavor Appropriate amount, appropriate amount of sorbic acid, appropriate amount of distilled water. Weigh the diluent in proportion, mix it evenly, and pass through an 80-mesh sieve; mix the prepared diluent with cyclobenzaprine hydrochloride, disintegrating agent, corrective agent, essence and preservative, add distilled water to granulate, Lubricant is added and compressed into tablets.
Embodiment 3
[0029] Cyclobenzaprine hydrochloride 30%, sucrose-lactose-mannitol 45% with a weight ratio of 3:2:5, microcrystalline cellulose 10%, carmellose calcium C 12%, sodium saccharin 1%, orange flavor Appropriate amount, appropriate amount of sorbic acid, appropriate amount of distilled water. Weigh the diluent in proportion, mix it evenly, and pass through an 80-mesh sieve; mix the prepared diluent with cyclobenzaprine hydrochloride, disintegrating agent, corrective agent, essence and preservative, add distilled water to granulate, Lubricant is added and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com