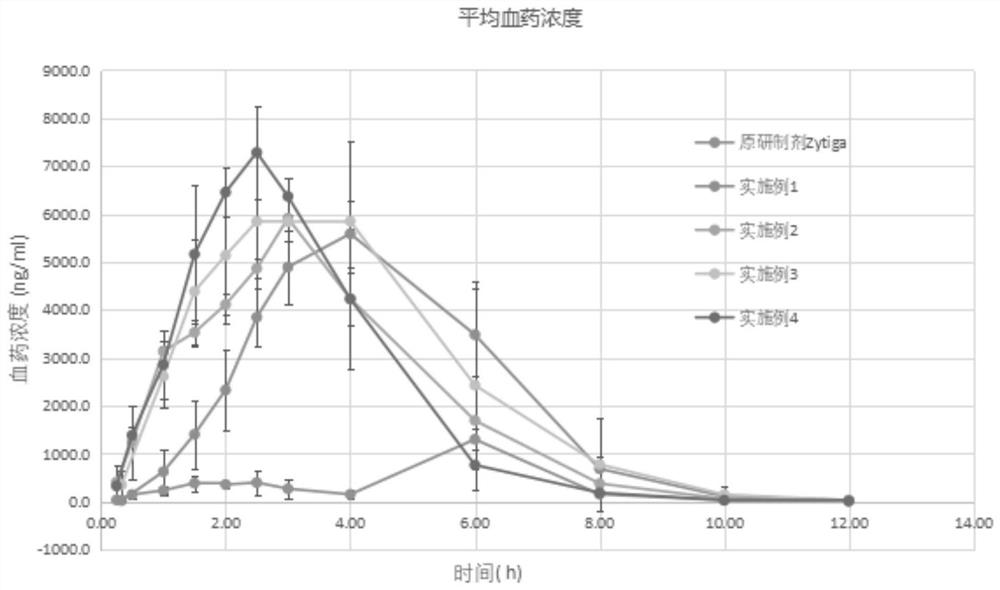
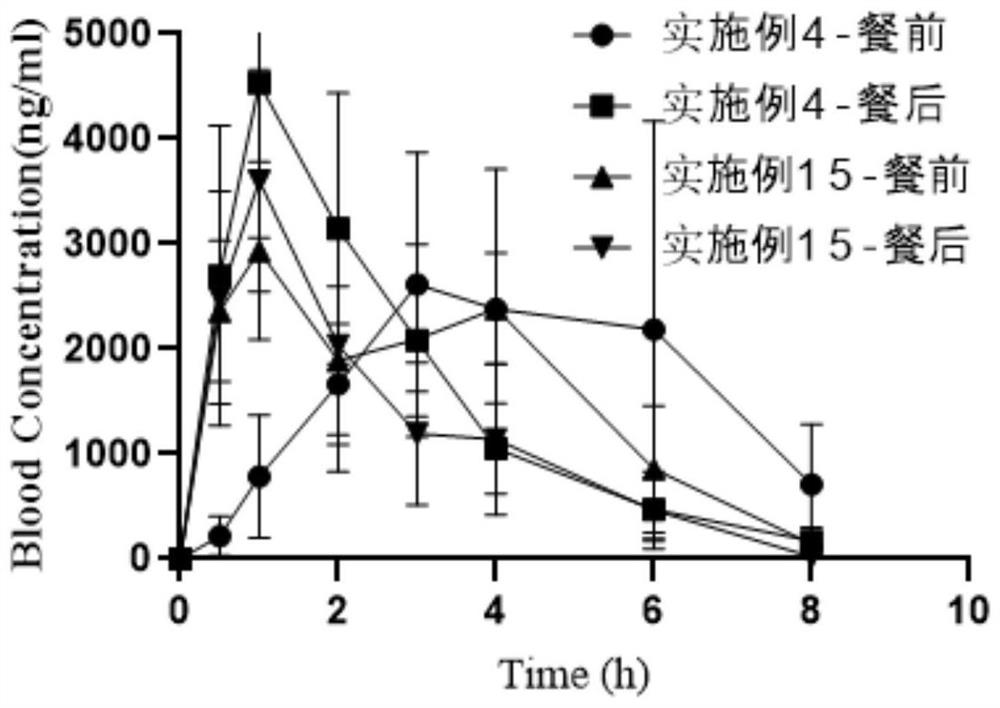
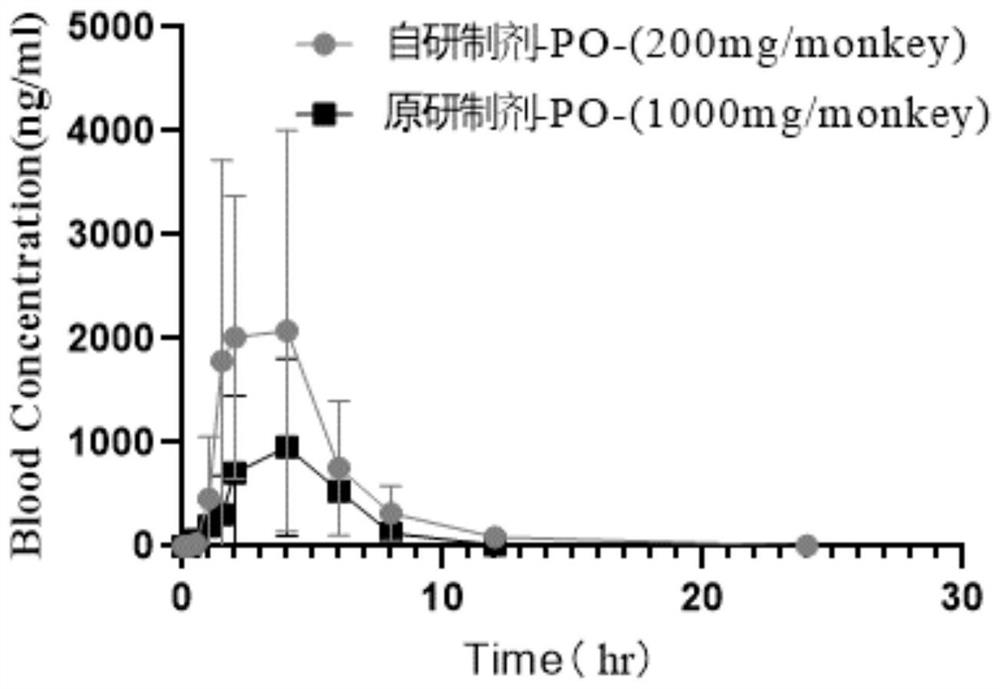
Abiraterone pharmaceutical composition with high drug loading capacity as well as preparation method and application of abiraterone pharmaceutical composition
A technology of abiraterone and abiraterone acetate, applied in the field of abiraterone pharmaceutical composition and preparation thereof, can solve the problems of product stability and curative effect, insufficient bioavailability, inconvenient administration, etc. Improve bioavailability and drug loading, realize large-scale industrial production, and eliminate the effect of blood drug concentration differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] The present embodiment provides a pharmaceutical composition containing abiraterone acetate, and the proportion of each component in the combination is calculated by mass fraction, including the following components:
[0089] Name Proportion (mass ratio)
[0090] Abiraterone acetate 28.6%
[0091] Oleic acid 71.4%
[0092] Preparation method: Add abiraterone acetate to oleic acid, stir or sonicate to dissolve it completely to form a homogeneous and clear solution.
[0093] The human body also secretes a large amount of bile salts in the gastrointestinal tract, and the phospholipids in the human body can self-emulsify oil-soluble substances. This embodiment is the simplest combination of the embodiments of the present invention.
Embodiment 2
[0095] The present embodiment provides a pharmaceutical composition containing abiraterone acetate, and the proportion of each component in the combination is calculated by mass fraction, including the following components:
[0096]
[0097] Preparation method: Mix oleic acid, polyoxyethylene 40 hydrogenated castor oil and propylene glycol evenly, then add abiraterone acetate, stir or sonicate to dissolve it completely to form a homogeneous and clear solution.
Embodiment 3
[0099] The present embodiment provides a pharmaceutical composition containing abiraterone acetate, and the proportion of each component in the combination is calculated by mass fraction, including the following components:
[0100]
[0101]
[0102] Preparation method: mix oleic acid, polyoxyethylene 35 castor oil and propylene glycol evenly, then add abiraterone acetate, stir or ultrasonicate to dissolve it completely to form a homogeneous and clear solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com