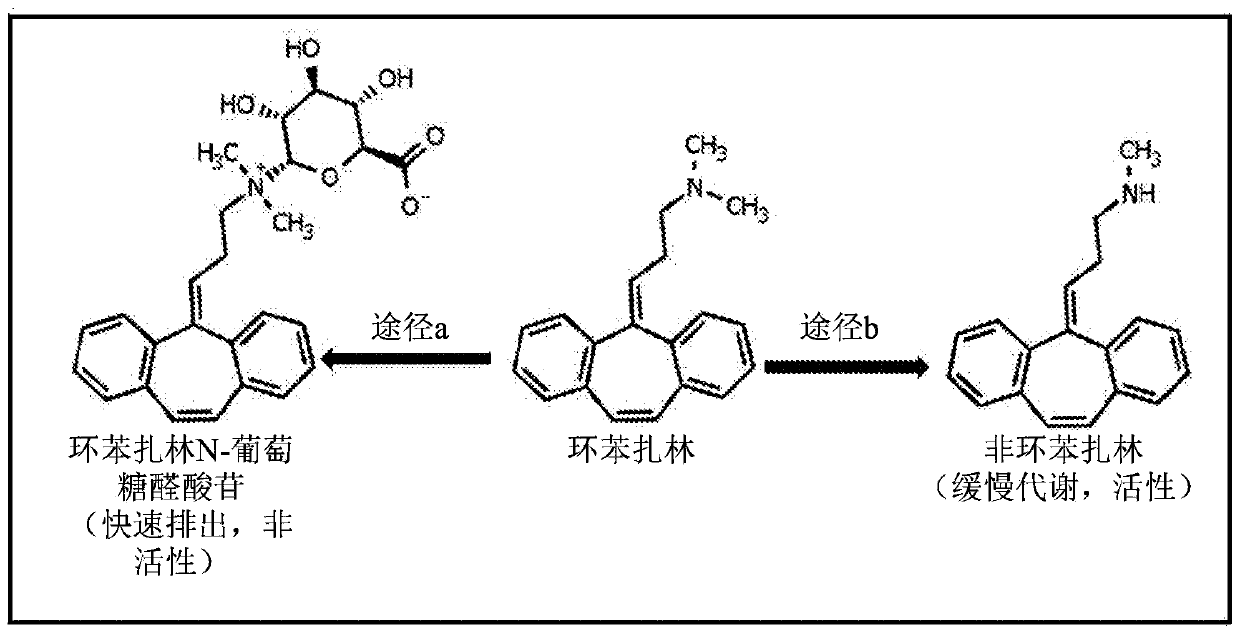
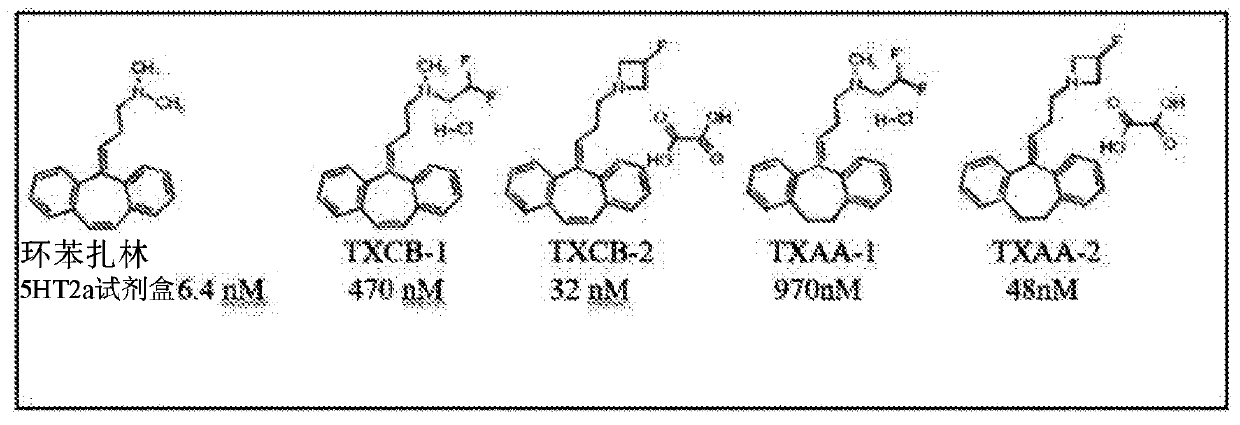
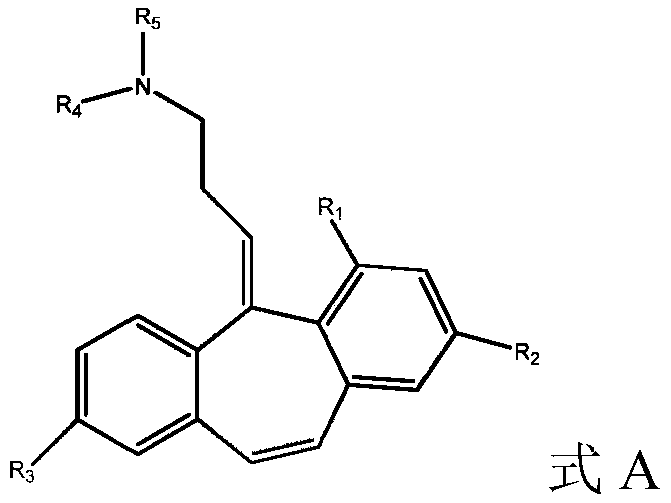
Analogs of cyclobenzaprine and amitryptilene
A technology of cyclobenzaprine and analogues, applied in the fields of drug combination, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0112] Purification of Trialkylamine Final Products in Examples 1-4 and 7-18. As free base, the trialkylamine final product can optionally be purified as follows: 1) using silica gel chromatography hexane-ethyl acetate, hexane-diethyl ether, dichloromethane-ethyl acetate, dichloromethane-methanol. Volatile trialkylamines such as triethylamine, trimethylamine or DIPEA can optionally be added to the solvent at 1-3% by volume to improve separation.
[0113] 2) Use reverse phase chromatography on C18 silica or phenyl silica. As a salt (including but not limited to oxalate, chloride or benzoate), the trialkylamine final product can be purified by recrystallization from a suitable solvent or solvent mixture including but not Limited to isopropanol, methanol, ethanol and their mixtures with ethyl acetate, chloroform and / or toluene.
example 1
[0114] Example 1 - TXAA-1, (2,2-difluoro-ethyl)-[3-(10,11-dihydro-dibenzo[a,d]cyclohepten-5-ylidene)-propane base]-methyl-amine; preparation of the hydrochloride salt
[0115]
[0116] Nortriptyline HCl (1.80 g, 6.00 mmol) was suspended in anhydrous THF (20 mL), and DIEA (2.30 mL, 13.2 mmol) was added at room temperature (RT) to obtain a suspension. The reaction mixture was briefly heated to gentle reflux, after which time a suspension remained. The suspension was cooled to 5 °C, 2,2-difluoro-ethyl trifluoromethanesulfonate (1.414 mL, 6.60 mmol) was added dropwise at 5 °C, then the reaction mixture was allowed to warm slowly to RT, and at RT Stirred at low temperature for 14 hours, after which an amber solution with a small amount of suspension was obtained. The solvent was evaporated in vacuo to give a solid which was washed with diethyl ether (Et 2 O) (200mL) extracted, washed with water (40mL), brine (40mL), washed with MgSO 4 After drying, the solvent was evaporated...
example 2
[0119] Example 2 - TXCB-1, (3-dibenzo[a,d]cyclohepten-5-ylidene-propyl)-(2,2-difluoro-ethyl)-methyl-amine; salt Salt preparation
[0120]
[0121] Norcyclobenzaprine (1.57 g, 6.00 mmol) was suspended in anhydrous tetrahydrofuran (THF) (20 mL), and N,N-diisopropylethylamine (DIEA) (1.25 mL, 7.20 mmol) was added at RT to obtain a suspension. The reaction mixture was briefly heated to gentle reflux to give a cloudy solution which was cooled to 5°C and 2,2-difluoro-ethyl trifluoromethanesulfonate (1.414 mL, 6.60 mmol), the reaction mixture was then allowed to warm slowly to RT and stirred at RT for 15 hours, after which a suspension was obtained. The solvent was evaporated in vacuo to give an oil which was washed with Et 2 O (120mL) extracted, washed with water (20mL), brine (20mL), washed with MgSO 4 dry. The solvent was evaporated in vacuo to give an oil which was dissolved in DCM (6 mL) and passed through SiO with Hex-EtOAc 2 Purification by chromatography gave an ambe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com