A kind of cyclobenzaprine hydrochloride sustained-release preparation
A technology for cyclobenzaprine hydrochloride and sustained-release preparations, which is applied in the direction of pill delivery, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of short half-life of cyclobenzaprine hydrochloride, frequent medication, Affect the patient's medication compliance and other issues, to achieve the effect of good stability and ideal release speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 cyclobenzaprine hydrochloride bag chip
[0022] According to the formula (g / tablet) in Table 1, the inner chip was pressed first, then coated, and finally the coated chip was pressed to prepare cyclobenzaprine hydrochloride coated chip Examples 1-7.
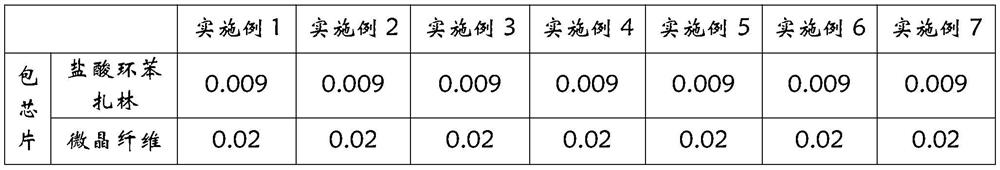
[0023] Table 1 Cyclobenzaprine hydrochloride packet chip formula (1)
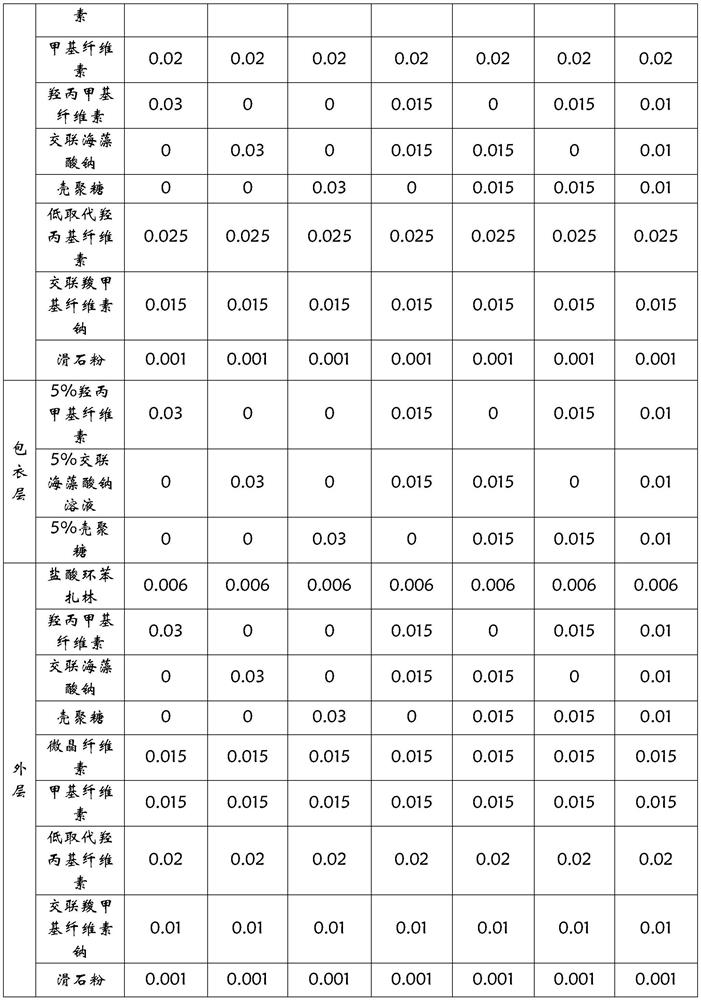
[0024]
[0025]
Embodiment 2
[0026] The screening of embodiment 2 cyclobenzaprine hydrochloride package chip sustained-release agent
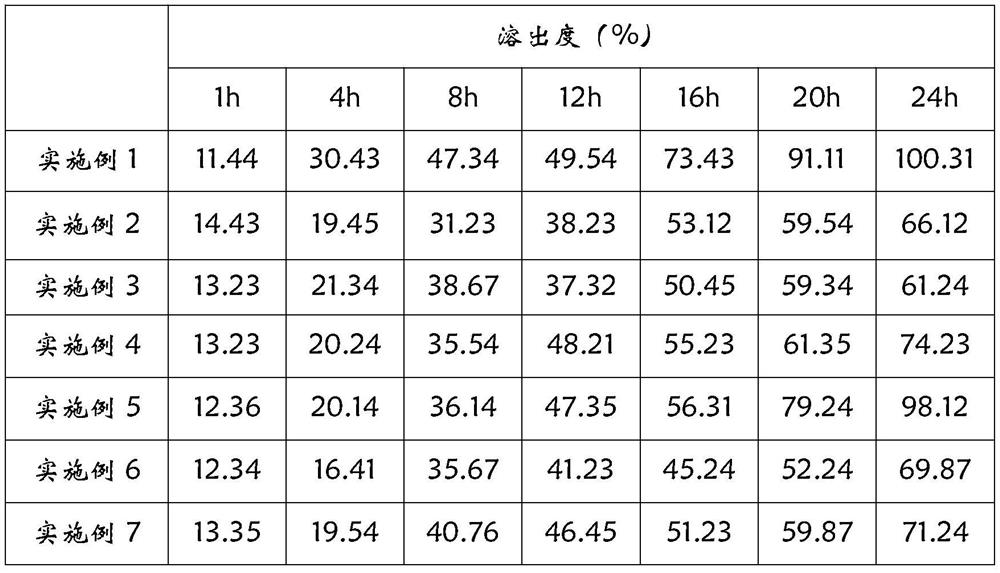
[0027] The cyclobenzaprine hydrochloride-coated chips of Examples 1 to 7 were subjected to a dissolution test. The test method was blue-turning method, the rotation speed was 50 rpm, the test solution was 900 mL of water, and the test temperature was 37±0.5° C. The results are shown in Table 2.
[0028] Table 2 Dissolution test results of cyclobenzaprine hydrochloride package chip of different sustained-release materials
[0029]
[0030] According to the above formula, it can be seen that the cyclobenzaprine hydrochloride-coated chip of the present invention is provided with the same slow-release agent in the inner chip and the outer layer, and the coating layer also adopts the same slow-release agent. According to the dissolution test results, it can be seen that when hydroxypropylmethylcellulose is used alone as a sustained-release agent, the release rate is too fas...
Embodiment 3
[0031] The preparation of embodiment 3 cyclobenzaprine hydrochloride bag chip
[0032] Select cross-linked sodium alginate and chitosan as sustained release agent, according to the formula (g / tablet) of table 3, first compress inner chip, then coat, finally compress and wrap chip, prepare cyclobenzaprine hydrochloride bag chip embodiment 8 to 10.
[0033] Table 3 Cyclobenzaprine hydrochloride packet chip formula (two)
[0034]
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com