A kind of ceftizoxime sodium liposome composition for injection and preparation method thereof
The technology of ceftizoxime sodium and liposome is applied in the field of ceftizoxime sodium liposome composition for injection and preparation thereof, and can solve the problems of high leakage rate and instability of ceftizoxime sodium liposome, etc. Achieving safe and effective clinical use, good stability and targeted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
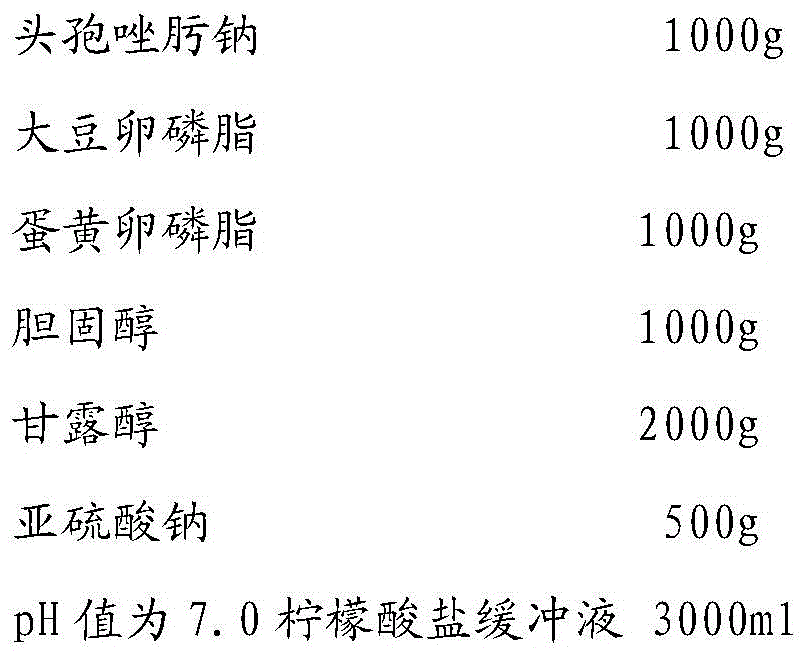
[0027] prescription
[0028]
[0029]
[0030] Preparation Process:
[0031] 1) Dissolve soybean lecithin, egg yolk lecithin and cholesterol in 800ml of isopropanol, stir and mix evenly, heat up and evaporate to completely remove isopropanol, and obtain a phospholipid film;
[0032] 2) Add 1500ml of citrate buffer solution to the phospholipid film in step 1), if necessary, adjust the pH to 7.0 with sodium hydroxide solution and hydrochloric acid, stir to hydrate the phospholipid film, then high-speed homogeneous emulsification, microporous membrane filtration , to obtain the filtrate, add water for injection until the filtrate is 2000ml;
[0033] 3) Add ceftizoxime sodium, mannitol, and vitamin E to the filtrate of step 2), stir and emulsify homogeneously at high speed, filter, sterilize, freeze-dry and subpackage the filtrate, or fill the filtrate in vials and freeze-dry , obtain ceftizoxime sodium liposome lyophilized preparation finished product, ceftizoxime sodium ...
Embodiment 2
[0035] prescription
[0036]
[0037] Preparation Process:
[0038] 1) Dissolve soybean lecithin, egg yolk lecithin and cholesterol in 1800ml of chloroform: ethanol in a mixed solvent of 1:3, stir and mix evenly, heat up and evaporate to completely remove chloroform and ethanol to obtain a phospholipid film;
[0039] 2) Add 3000ml of citrate buffer solution to the phospholipid film in step 1), adjust pH to 7.0 with sodium hydroxide solution and hydrochloric acid if necessary, stir to hydrate the phospholipid film, then high-speed homogeneous emulsification, microporous membrane filtration,
[0040] Obtain filtrate, add water for injection until filtrate is 4000ml;
[0041] 3) Add ceftizoxime sodium, mannitol, and sodium sulfite to the filtrate of step 2), stir and emulsify at a high speed, filter, sterilize, freeze-dry and repack the filtrate, or fill the filtrate in vials and freeze-dry. Obtain the finished ceftizoxime sodium liposome freeze-dried preparation, ceftizoxim...
Embodiment 3
[0043] prescription
[0044]
[0045] Preparation Process:
[0046] 1) Dissolving soybean lecithin, egg yolk lecithin and cholesterol in 6000ml of absolute ethanol, stirring and mixing evenly, heating up and evaporating to completely remove ethanol to obtain a phospholipid film;
[0047]2) Add 1500ml of citrate buffer solution to the phospholipid membrane in step 1), if necessary, adjust the pH to 6.5 with sodium hydroxide solution and hydrochloric acid, stir to hydrate the phospholipid membrane, then high-speed homogeneous emulsification, microporous membrane filtration , to obtain the filtrate, add water for injection until the filtrate is 2000ml;
[0048] 3) Add ceftizoxime sodium, mannitol, and sodium sulfite to the filtrate of step 2), stir and emulsify at a high speed, filter, sterilize, freeze-dry and repack the filtrate, or fill the filtrate in vials and freeze-dry. Obtain the finished ceftizoxime sodium liposome freeze-dried preparation, ceftizoxime sodium 0.5g / b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com