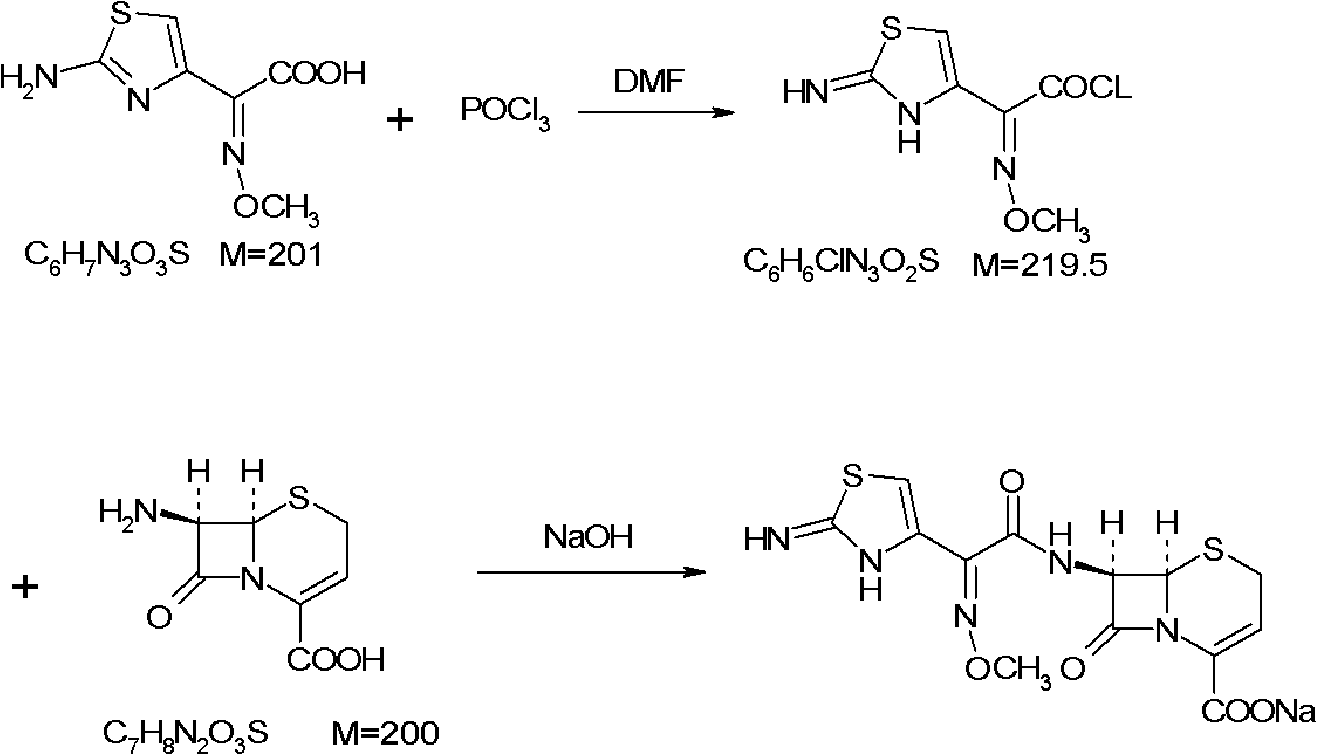
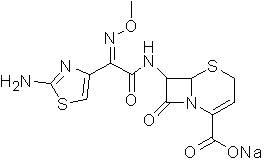
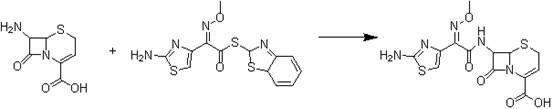
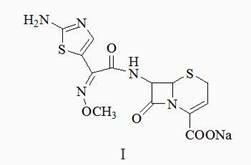
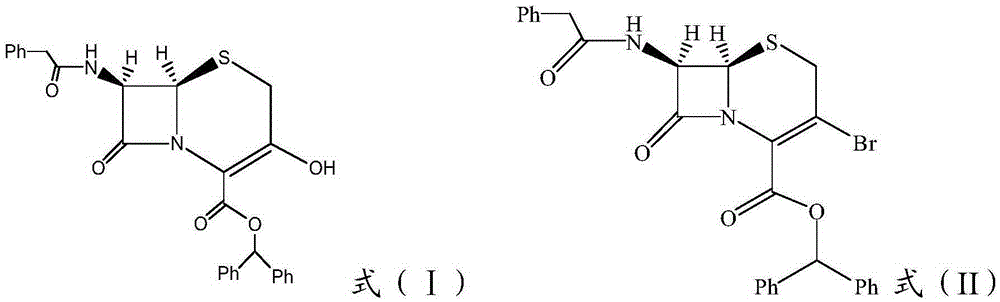
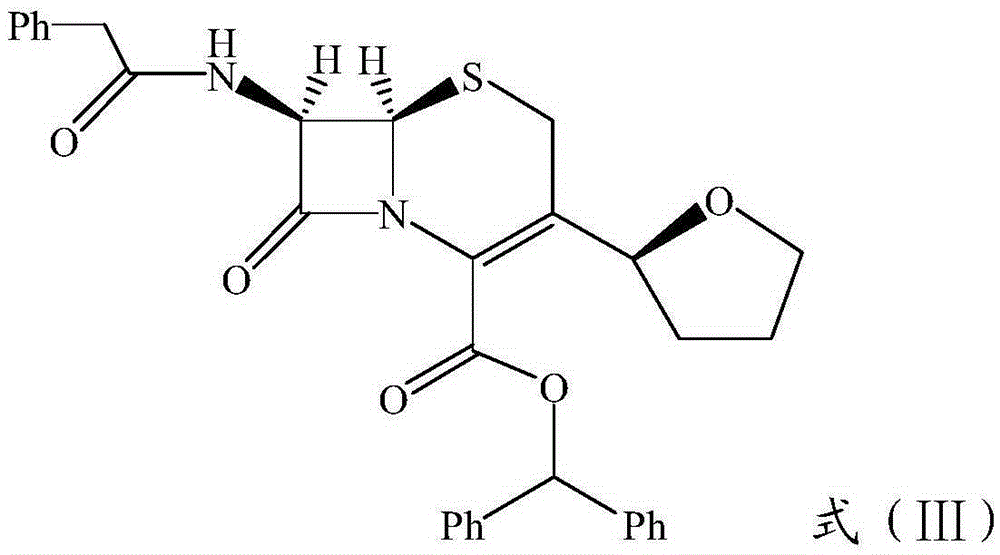
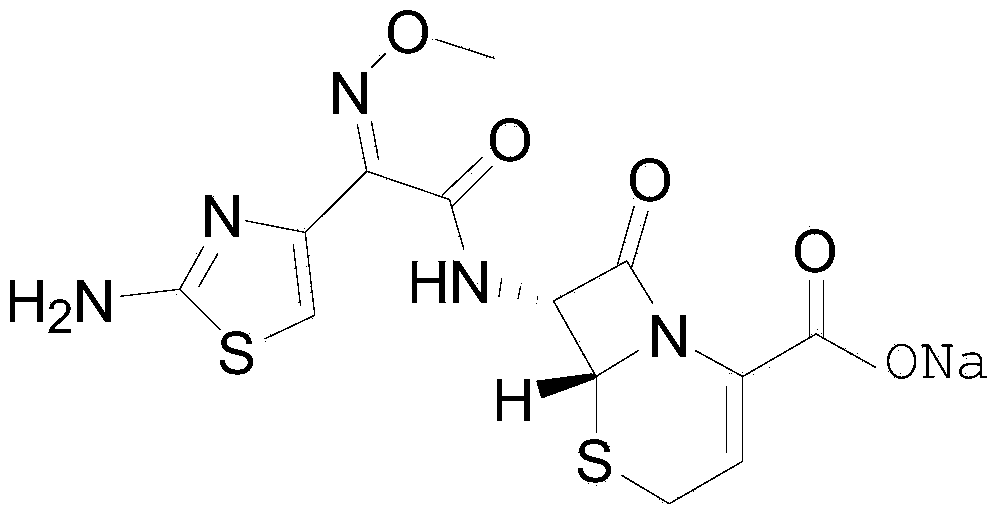
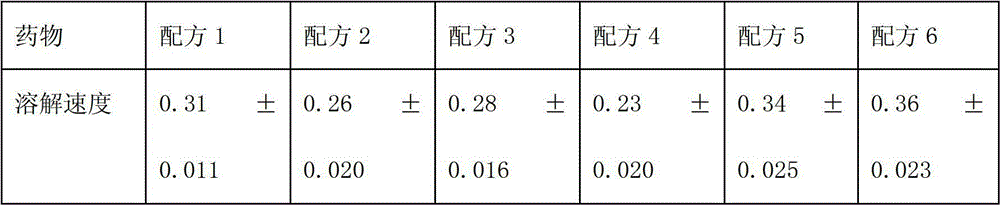
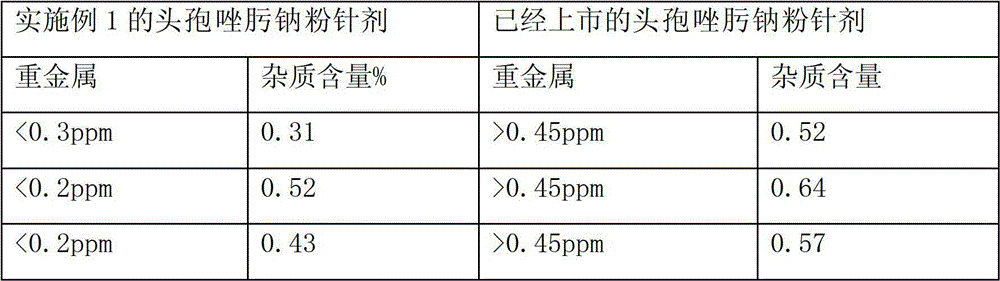
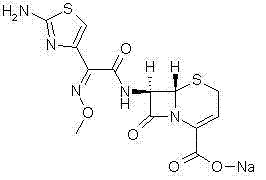
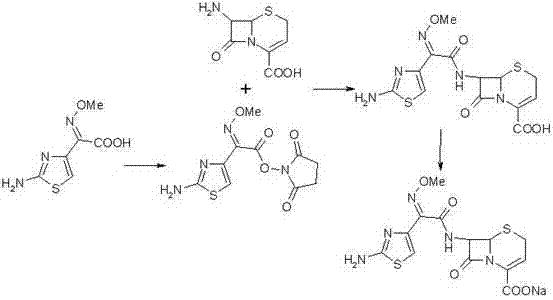
Patents
Literature
59 results about "Ceftizoxime Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
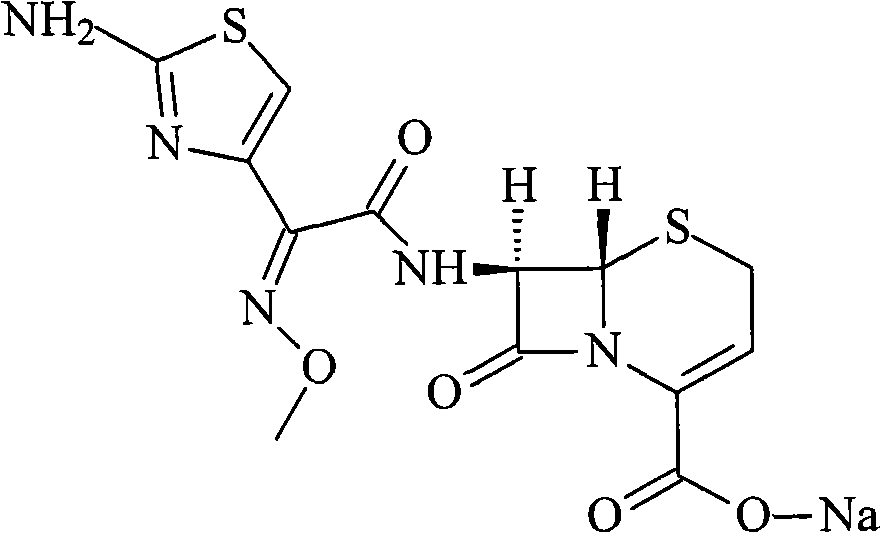
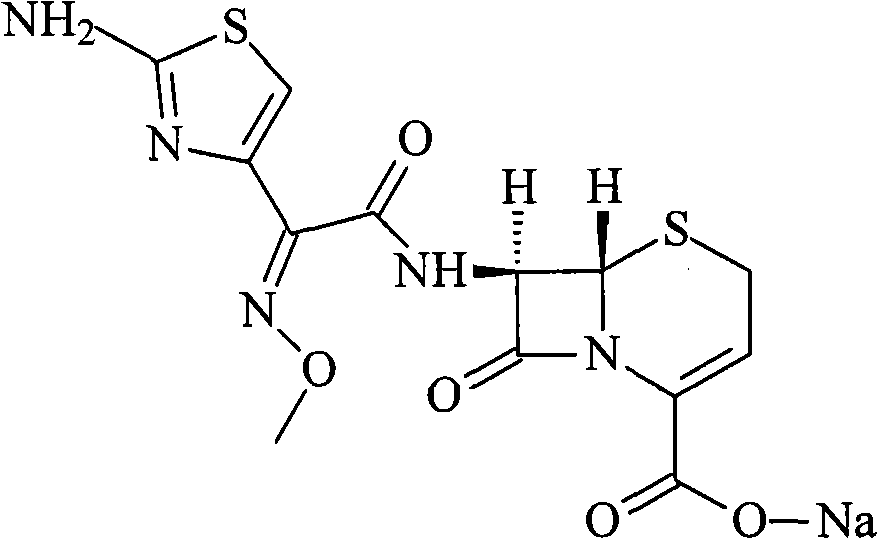
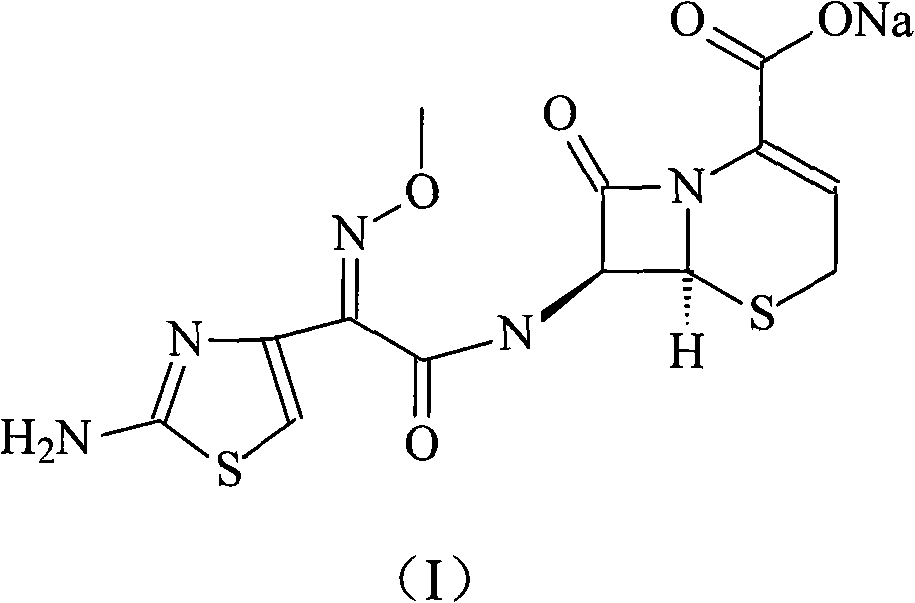
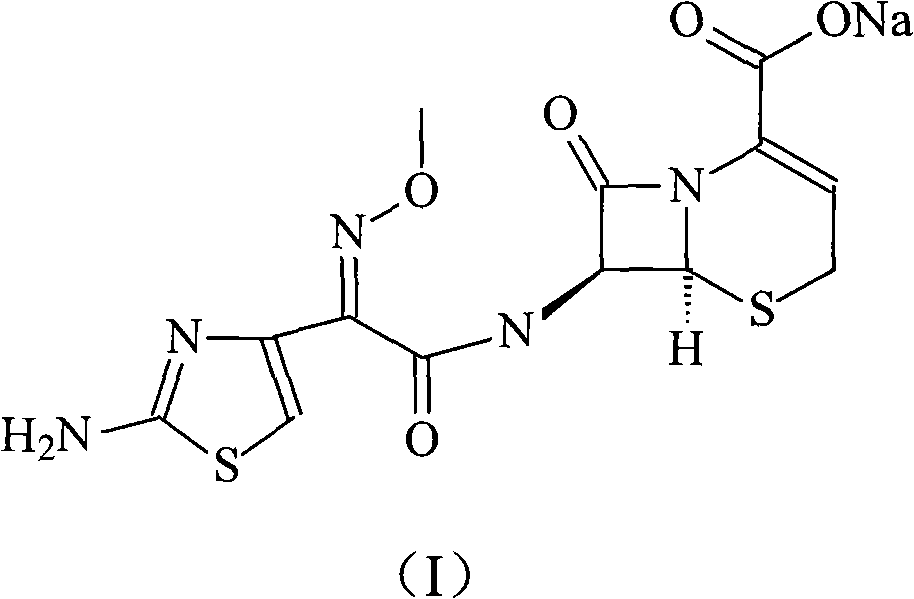
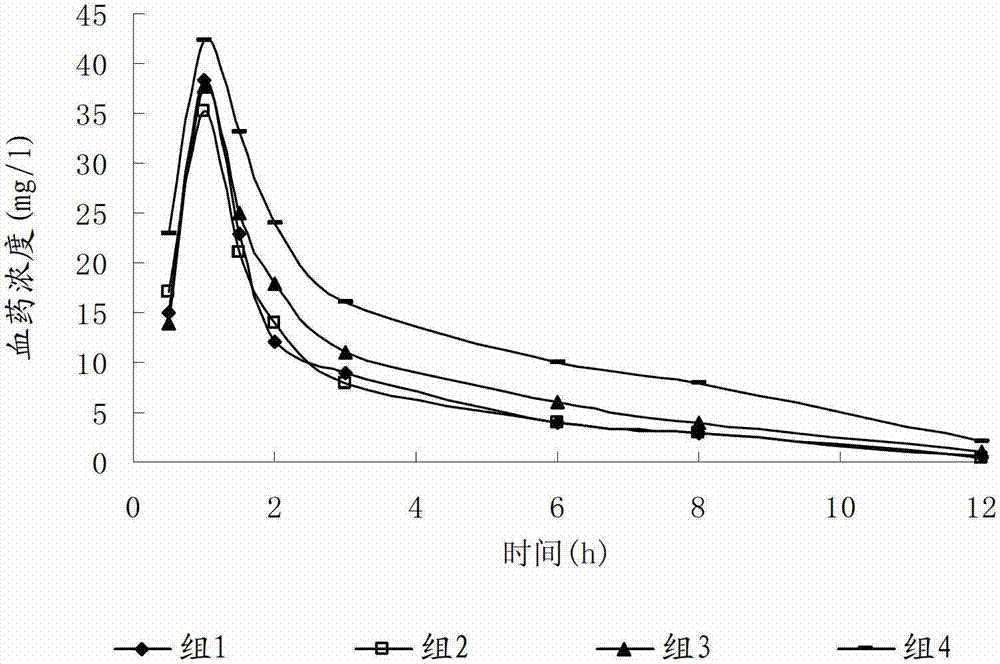
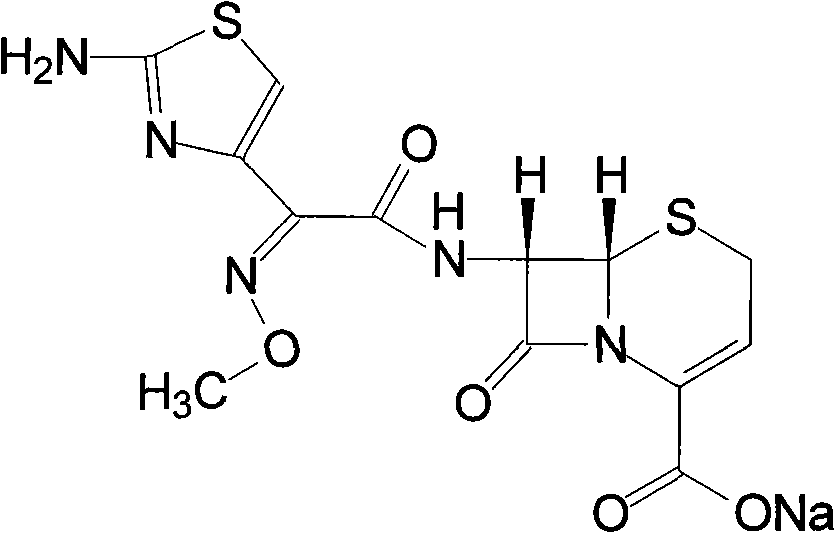
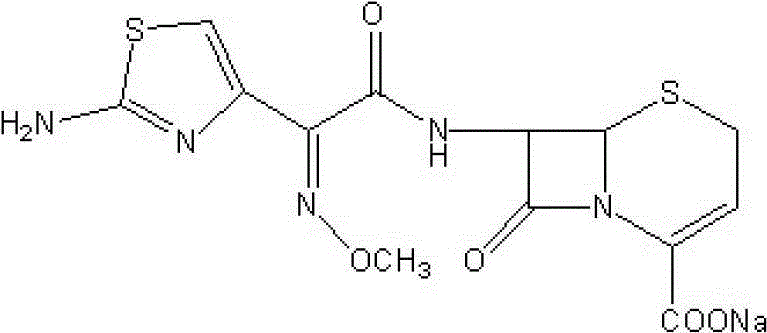
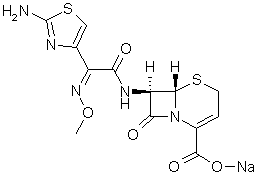
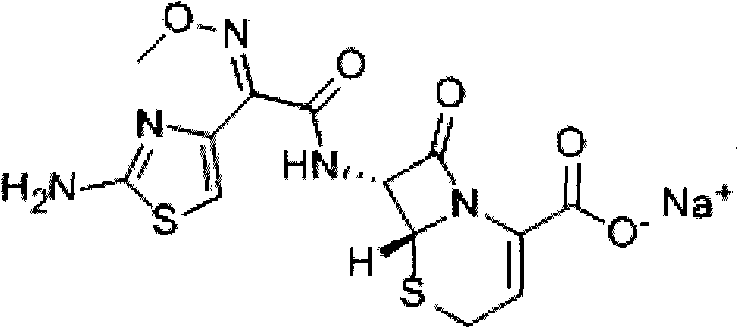
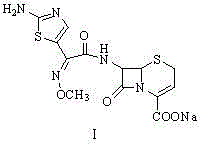
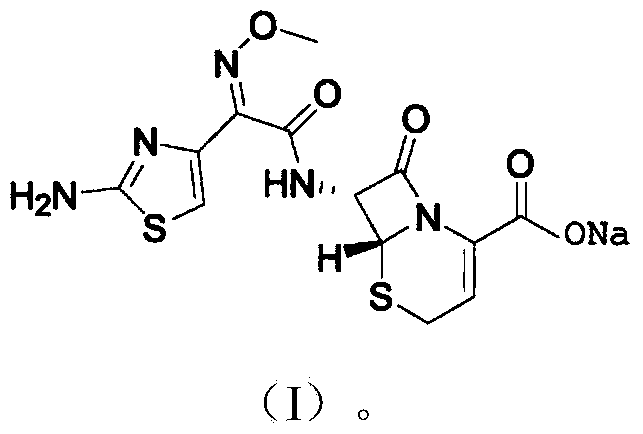
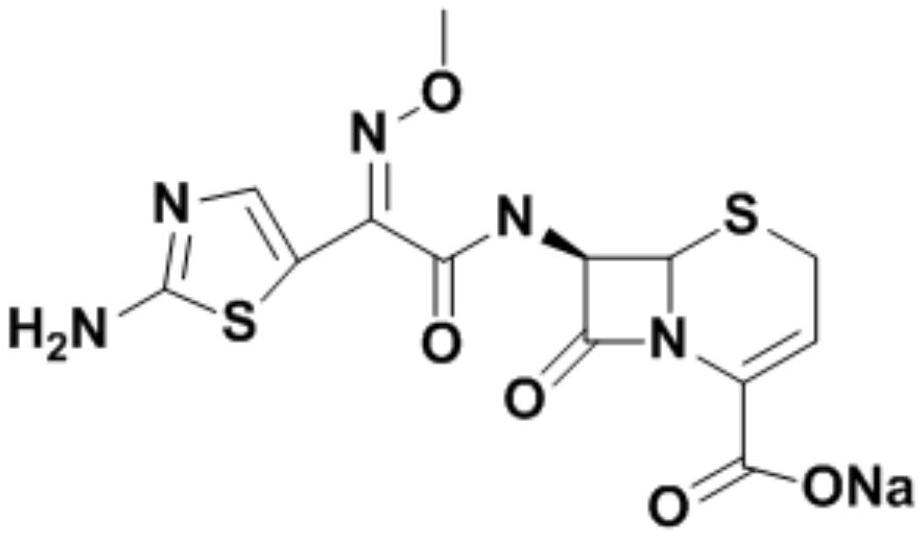
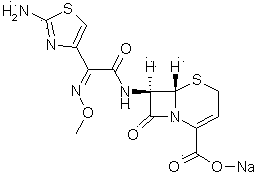
The sodium salt form of ceftizoxime and a semi-synthetic, broad-spectrum, beta-lactamase resistant, third-generation cephalosporin antibiotic with bactericidal activity. Ceftizoxime sodium inhibits bacterial cell wall synthesis by inactivating penicillin binding proteins (PBPs) thereby interfering with the final transpeptidation step required for cross-linking of peptidoglycan units which are a component of the cell wall. Lack of cross-linking results in a reduction of cell wall stability and leads to cell lysis.
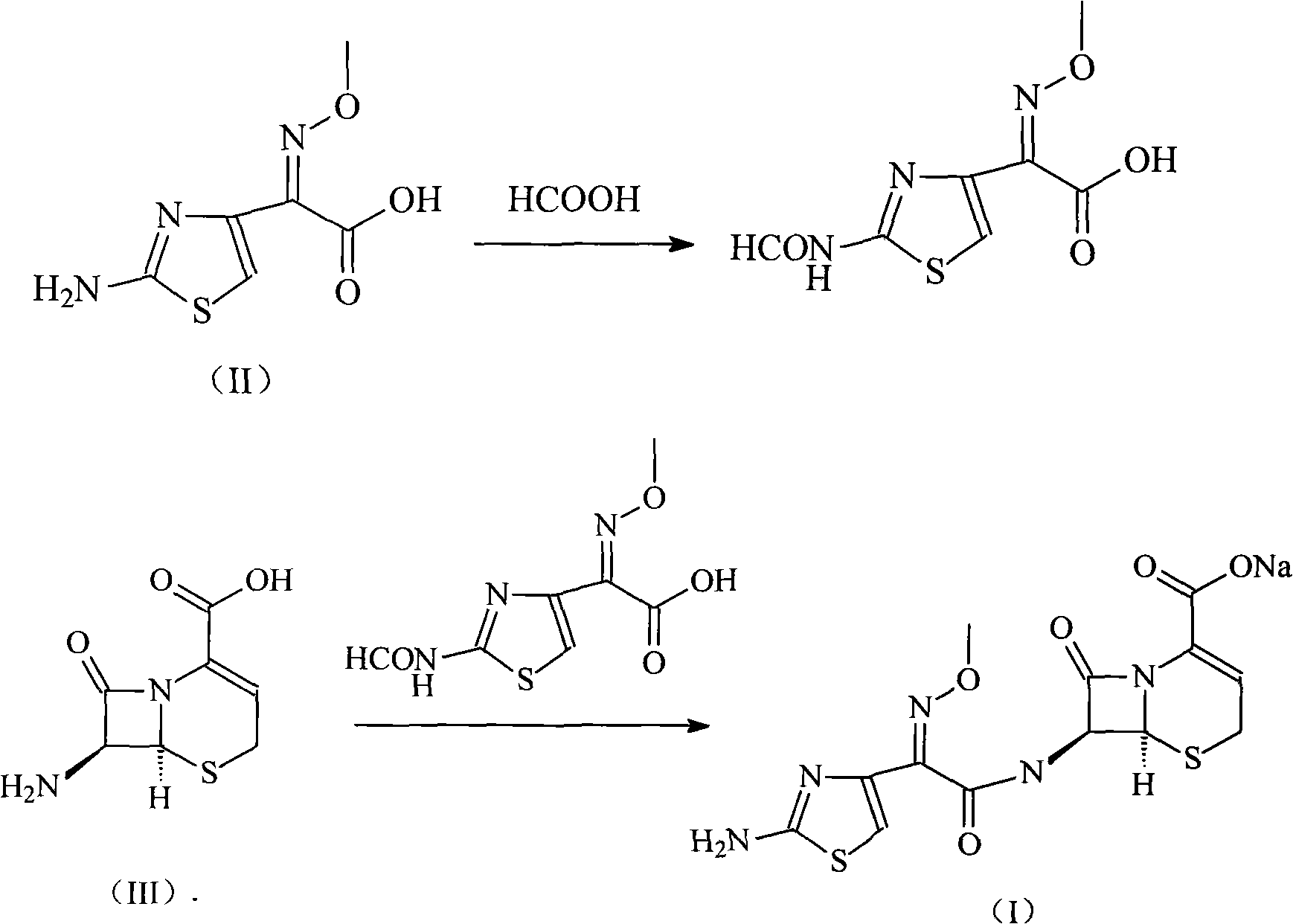
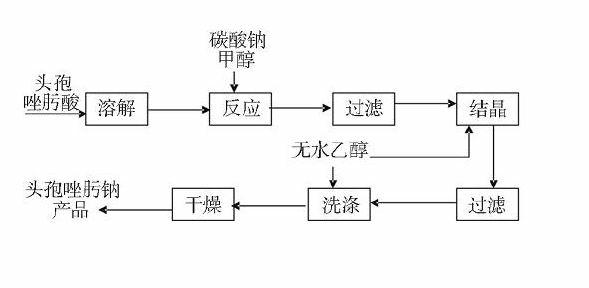
High-purity ceftizoxime sodium and preparation thereof
InactiveCN101348492AHigh purityNo pollution in the processAntibacterial agentsPowder deliveryCeftizoxime SodiumInorganic chemistry
Owner:HAINAN LINGKANG PHARMA CO LTD
Ceftizoxime sodium drug injection powder and preparation method thereof, as well as synthetic method of bulk drug ceftizoxime sodium
ActiveCN101606910AUniform colorHigh purityAntibacterial agentsOrganic active ingredientsDrug injectionCLARITY
The invention relates to a ceftizoxime sodium drug injection powder and a preparation method thereof, as well as a synthetic method of bulk drug ceftizoxime sodium. The ceftizoxime sodium drug injection powder consists of 100% ceftizoxime sodium, wherein the ceftizoxime sodium is pretreated, and the pretreatment is aseptic refining and / or grinding. The ceftizoxime sodium drug has the advantages of high purity, almost no impurity, better and more stable quality, better clarity and the like; and the synthetic method of bulk drug ceftizoxime sodium has lower bulk cost, less synthesis technology difficulty, mild reaction condition, stable and reliable yield and quality, and high purity and yield of products.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Preparation method of ceftizoxime sodium
InactiveCN102603771ASimple manufacturing processMild reaction conditionsOrganic chemistryCeftizoxime SodiumSolvent
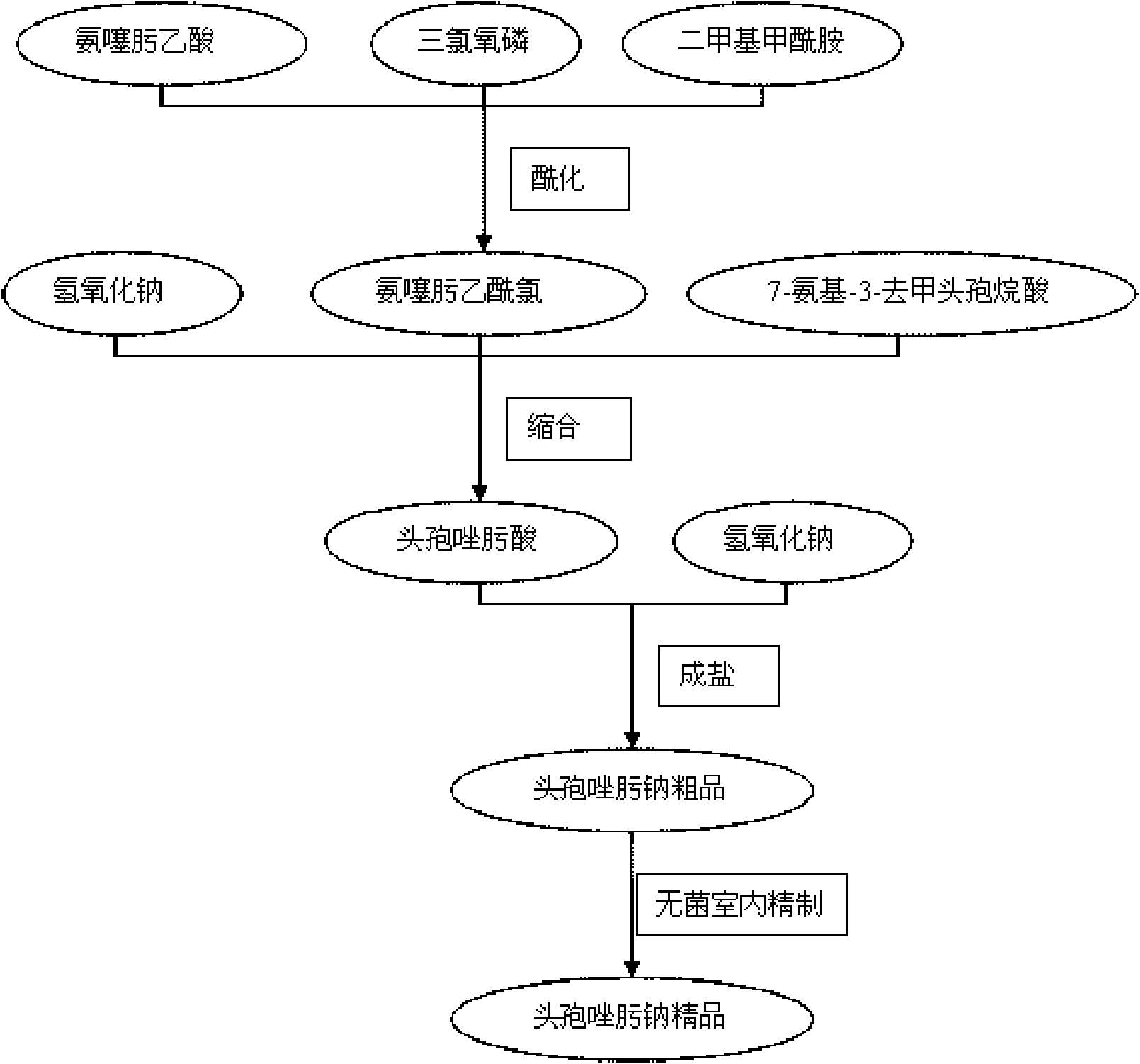
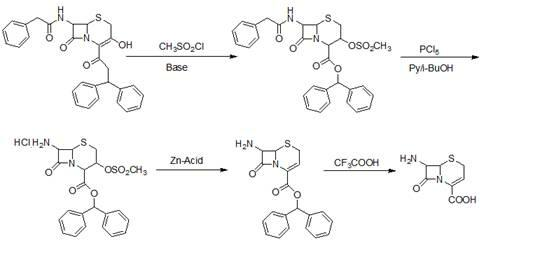
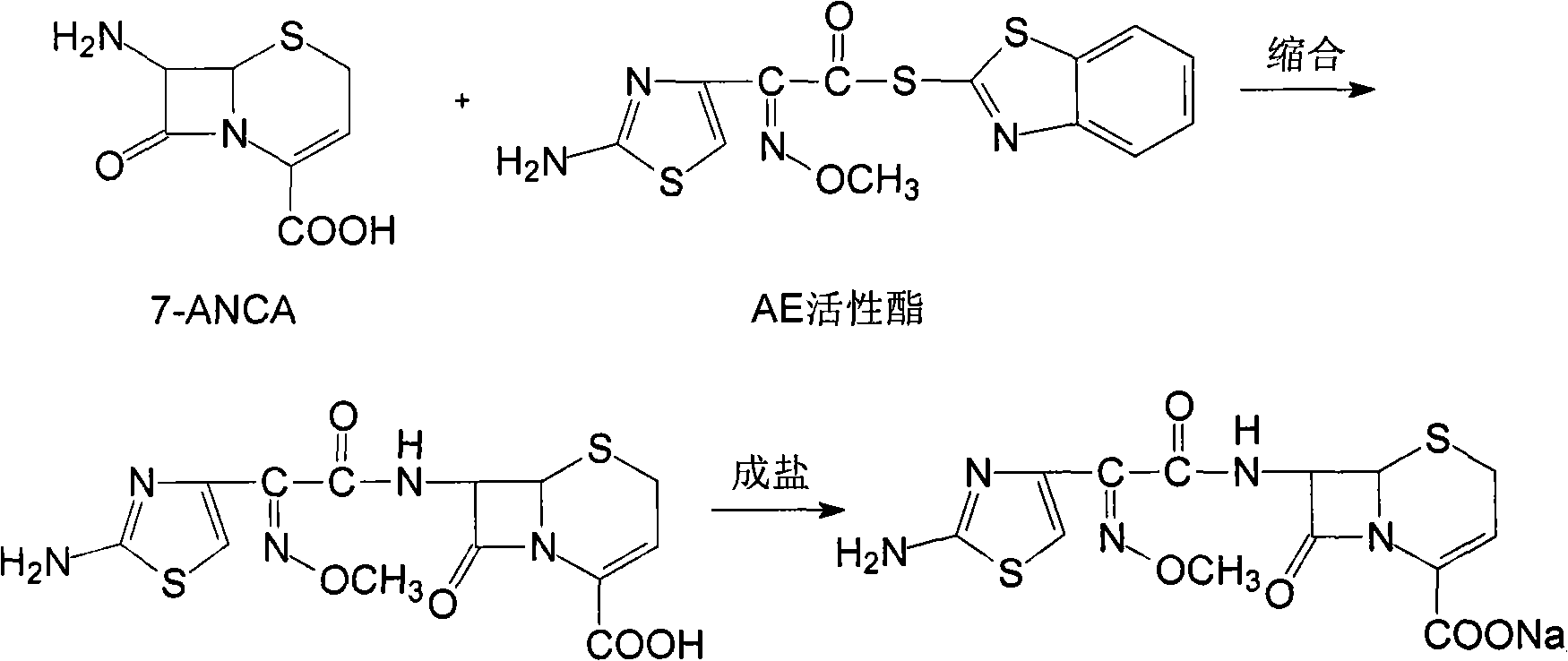
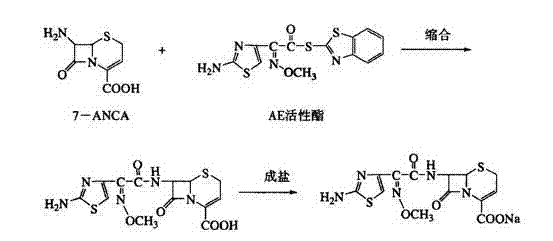
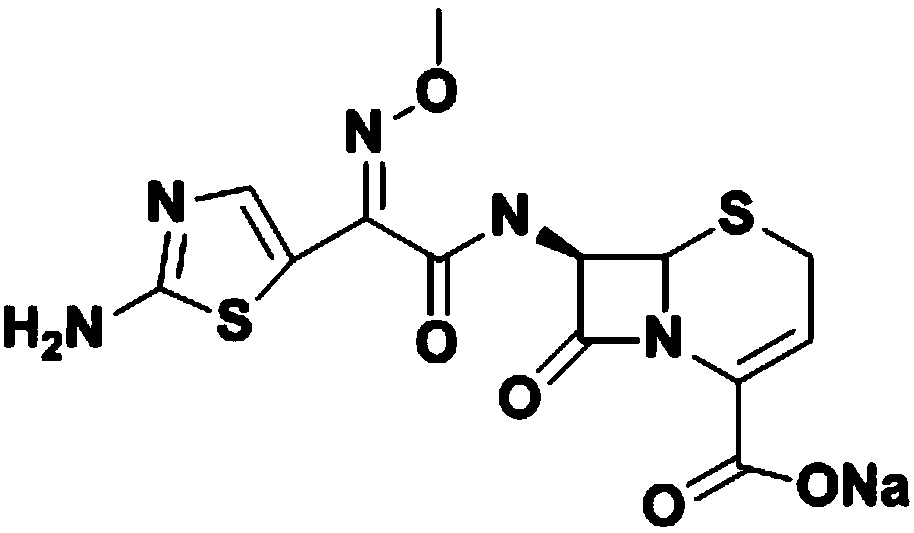
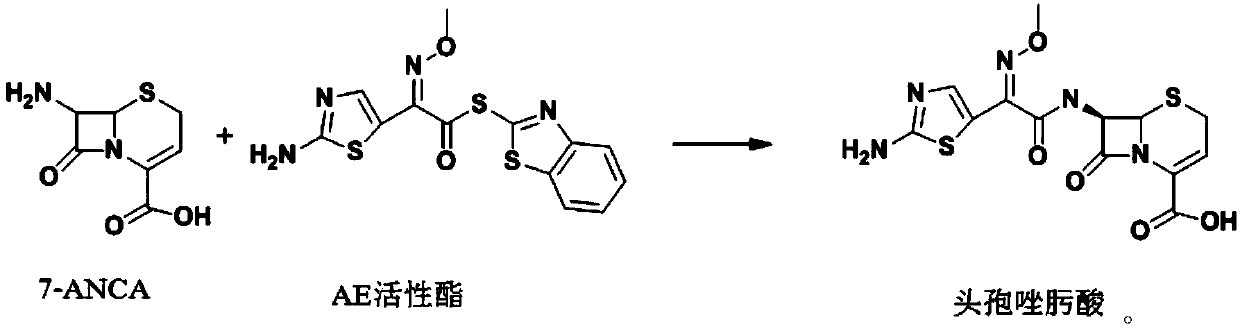
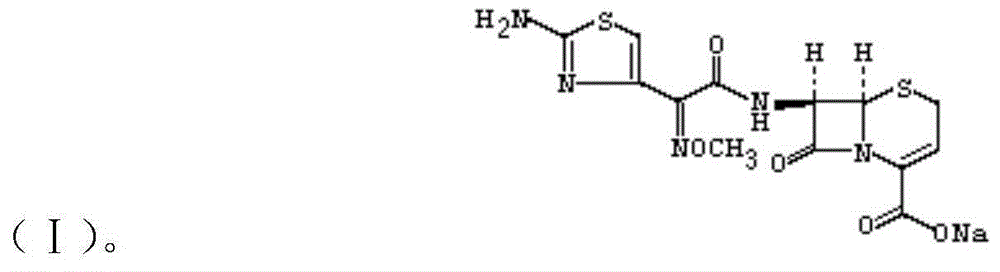
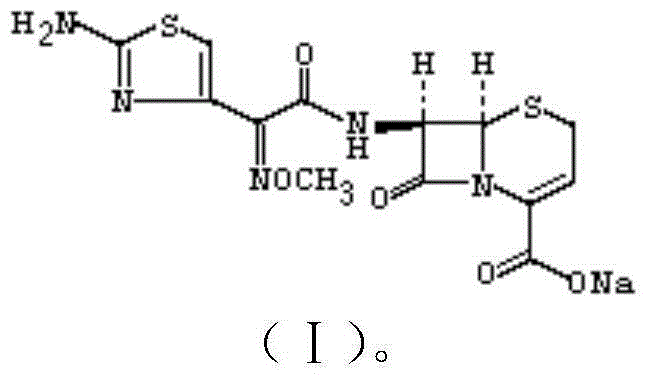
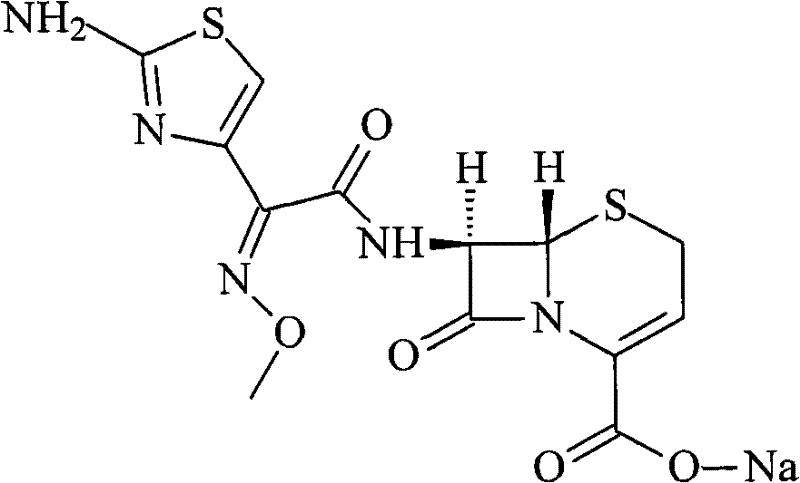
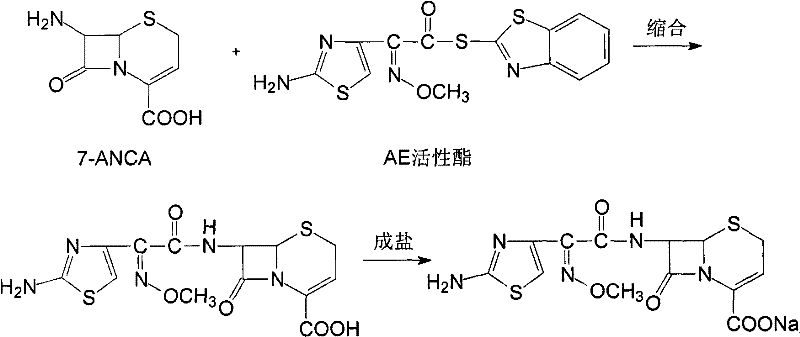
The invention discloses a preparation method of ceftizoxime sodium. The method comprises the following steps of: condensing 7-ANCA serving as a raw material with 2-(2-amino-4-thiazolyl)-2-methoxyimine-acetyl-benzothiazole thioester (AE-active ester) in a solvent in the presence of an alkali to obtain a ceftizoxime acid intermediate; and (2) reacting the ceftizoxime acid intermediate with sodium hydrate serving as a salting agent to obtain ceftizoxime sodium. Compared with the prior art, the method has the advantages of easy, convenient and practicable preparation process, mild reaction conditions, low cost, high yield, high product purity and suitability for industrial production.
Owner:苏州盛达药业有限公司
Preparation method of ceftizoxime sodium
InactiveCN102219794AEasy to operateLow costOrganic chemistryBulk chemical productionBenzeneCeftizoxime Sodium
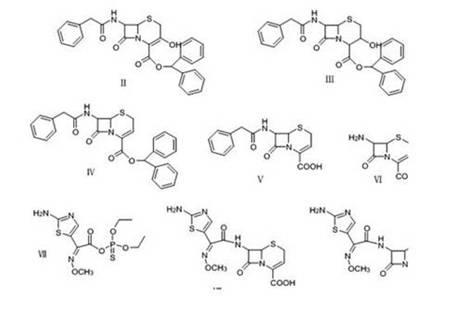
The invention relates to a preparation method of ceftizoxime sodium. The method comprises the following steps: based on 7-acetamido-3-hydroxy-3-cephalosporin-4-formic acid-diphenyl methyl ester (II) sas a starting material, carrying out reduction, sulfoacid esterification, de-exterification sulfonation and 4-site and 7-site protective groups removal so as to obtain an important intermediate, namely, 7-amino-3-free-3-cephalosporin-4-formic acid (VI, 7-ANCA); reacting 7-amino-3-free-3-cephalosporin-4-formic acid (VI, 7-ANCA) with an active ester so as to obtain ceftizoxime acid (VIII); and generating the product, namely, ceftizoxime sodium (I), from the ceftizoxime acid (VIII) in the presence of a salt forming agent. The method for preparing ceftizoxime sodium is simple in operation, low in cost and suitable for industrial production, and the total yield can reach 52.9%. The product is white or light yellow, and rectification is not needed, thereby ensuring the quality and yield of the product.
Owner:TIANJIN GREENPINE PHARMA
Cefuroxime sodium compound and new preparation method thereof
InactiveCN102079750AHigh purityIncrease contentAntibacterial agentsOrganic chemistryActivated carbonUpper urinary tract infection
The invention provides a cefuroxime sodium compound and a new preparation method thereof. The method comprises the following steps: performing acid-base reaction, absorbing with activated carbon, and separating and purifying with a chromatographic column. By adopting the method, the purity and content of cefuroxime sodium can be greatly increased, the product quality of the preparation can be increased, the toxic or side effects can be reduced and the safety of the preparations of the medicines for curing respiratory tract infection and urinary tract infection can be ensured; and compared with the prior art, the method has the advantages of simple and practical technology, mild reaction conditions, low cost, high yield and high product purity and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
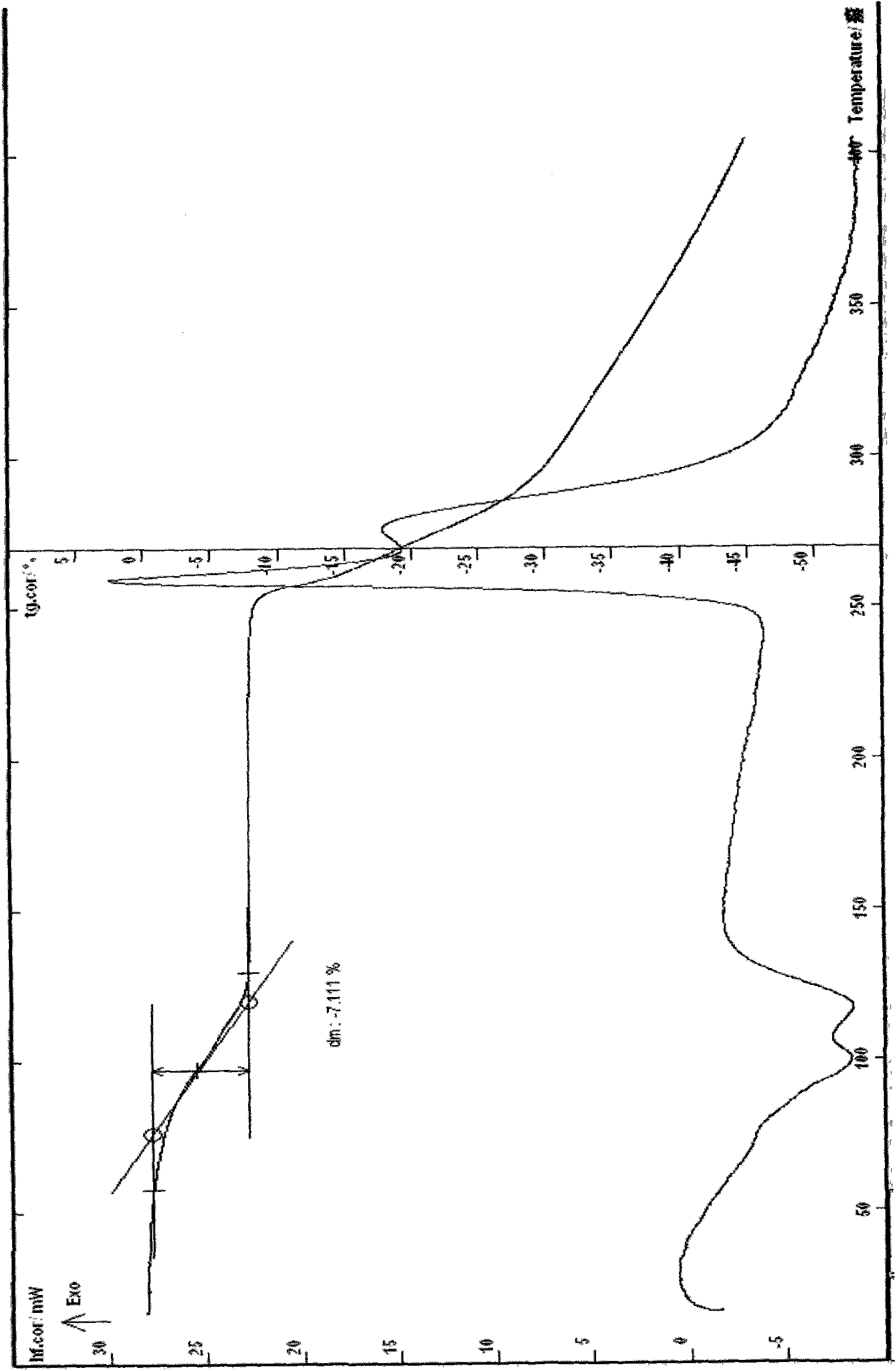
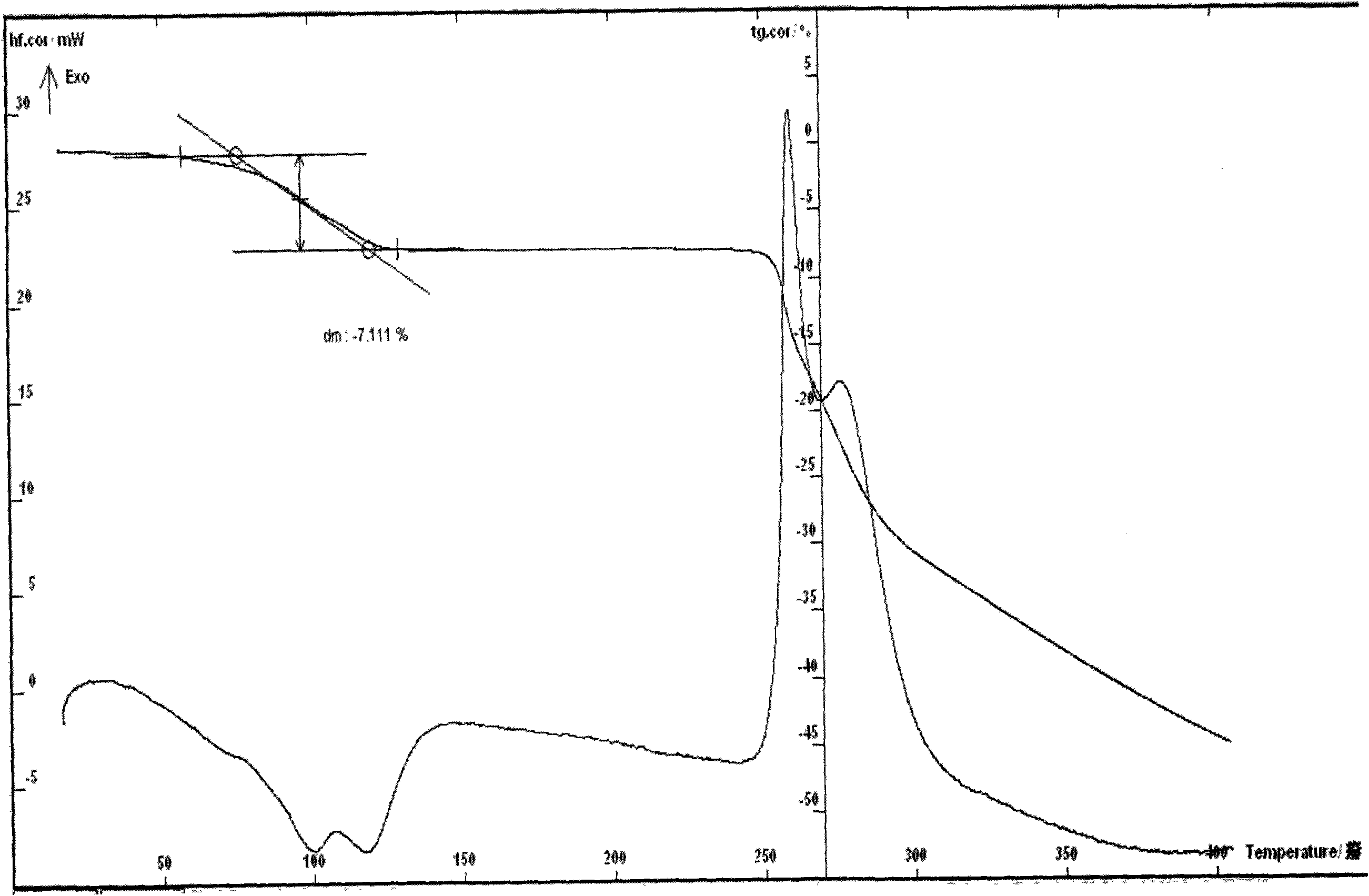
Ceftizoxime sodium crystalline hydrate and preparation method and application thereof
InactiveCN101781316AReduce humidityEasy to makeAntibacterial agentsPowder deliveryJoint infectionsBacilli
The invention relates to a ceftizoxime sodium crystalline hydrate and a preparation method and application thereof. The ceftizoxime sodium crystalline hydrate has high storage stability, and is suitable for preparing medicaments for treating or preventing diseases such as infection of respiratory systems, liver and biliary systems, five sense organs and urinary tracts of human bodes or animals, infection of abdominal cavity, infection of pelvic cavity, ichorrhemia, infection of skin tissues, and infection of bones and joint caused by gram positive or negative bacteria, meningitis caused by streptococcus pneumonia or haemophilus influenza, and simple gonorrhea.
Owner:刘力
Ceftizoxime sosium compound of new way
InactiveCN101671348AHigh purityHigh yieldAntibacterial agentsOrganic chemistryAcetic acidTriphenylphosphine oxide
The invention relates to a ceftizoxime sosium compound of a new way. Ceftizoxime sosium is prepared by firstly reacting a cefotaxime acid with a methanoic acid to generate 2-(2-formyl aminothiazole-4-radical)-2-methoxyl imine acetic acid, then adding 7-amino-3-nor-3-cephem-4-carboxylic acid, taking triphenylphosphine oxide and triphosgene as catalysts, stirring and reacting, and regulating an acid-base pH value.
Owner:HAINAN LINGKANG PHARMA CO LTD
Pharmaceutical composition of injection ceftizoxime sodium and compound amino acid injection
InactiveCN103110640AIncrease profitIncrease savingsAntibacterial agentsOrganic active ingredientsMedicinePharmaceutical drug
The invention relates to a pharmaceutical composition of injection ceftizoxime sodium and compound amino acid injection and in particular relates to combined application package. The pharmaceutical composition includes injection ceftizoxime sodium and compound amino acid injection. During a using process, the injeciton ceftizoxime sodium is dropped to the compound amino acid injection for intravenous infusion of 30 minutes to 2 hours. For the compatibility mixing of the injection ceftizoxime sodium and the compound amino acid injection, the combined application package disclosed by the invention is used for simplifying the steps and improving the safety. Moreover, the stability of the ceftizoxime sodium is greatly facilitated, and the clinical application quality and the bioavailability of the medicine are improved.
Owner:海南路易丹尼生物科技有限公司
Convenia synthetic method and Convenia sodium salt synthetic method
ActiveCN105254648ASimple reaction conditionsSimplified synthetic routeOrganic chemistryElectrophilic additionCeftizoxime Sodium
The invention discloses a Convenia synthetic method and a Convenia sodium salt synthetic method. A ceftizoxime sodium midbody is taken as the raw material and is subjected to bromination, electrophilic addition, nucleophilic substitution, deprotection, amidation and the like to obtain Convenia and Convenia sodium salt. According to the Convenia synthetic method, synthetic routes and steps are simplified through technology improvement and reaction condition improvement, and cost is saved. The Convenia synthetic method and the Convenia sodium salt synthetic method are easy to operate, mild in reaction condition, low in requirement for production equipment, and capable of creating sound conditions for industrialization. It shows through analysis and detection that a prepared product is high in quality and can meet the requirements of industrial and agricultural production.
Owner:GUANGDONG WENS DAHUANONG BIOTECH +1
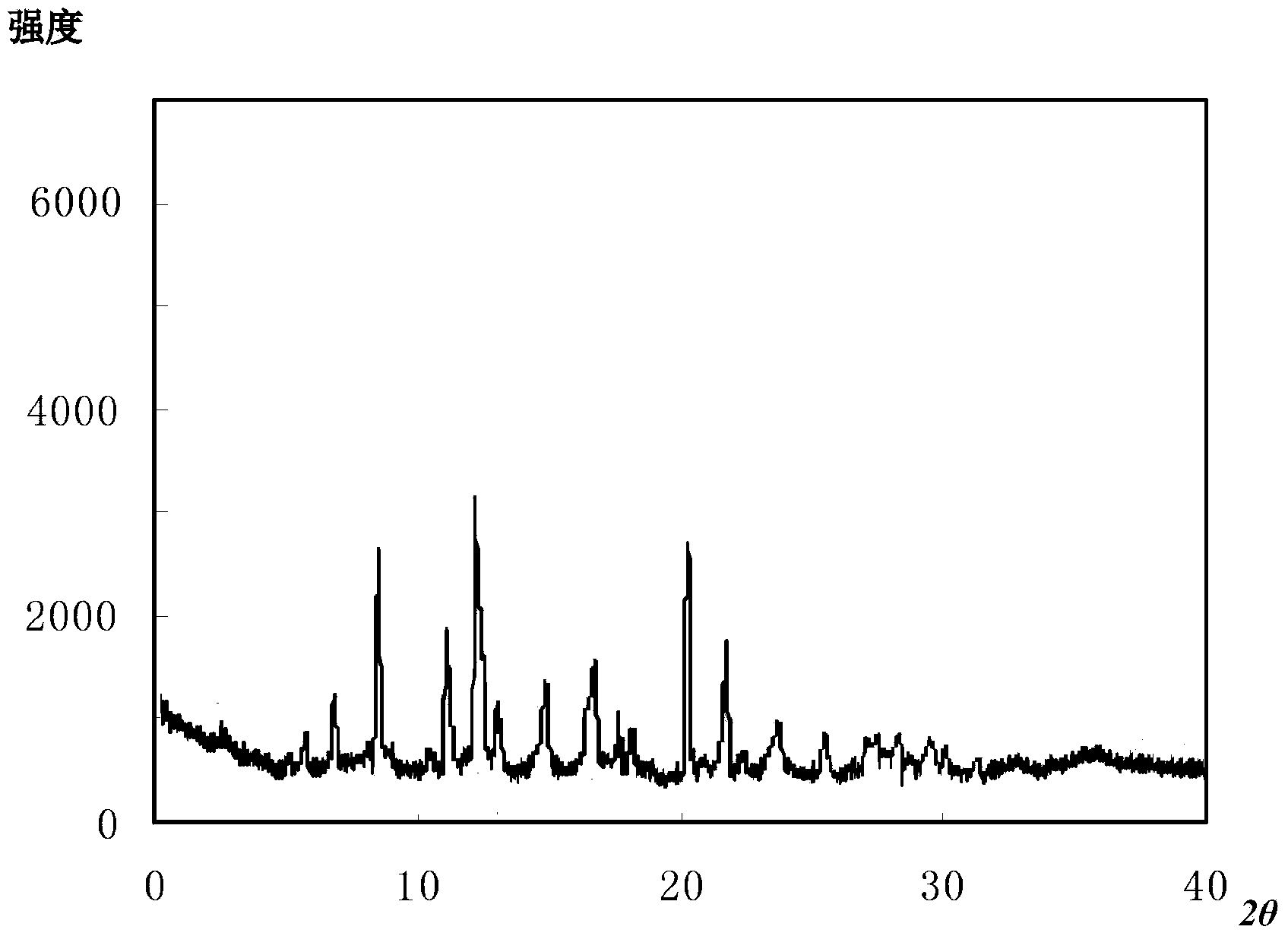
Ceftizoxime sodium compound crystal form, and preparing method and pharmaceutical preparation thereof
ActiveCN103524532AUniform particle size distributionImprove liquidityAntibacterial agentsOrganic active ingredientsCeftizoxime SodiumPowder diffraction
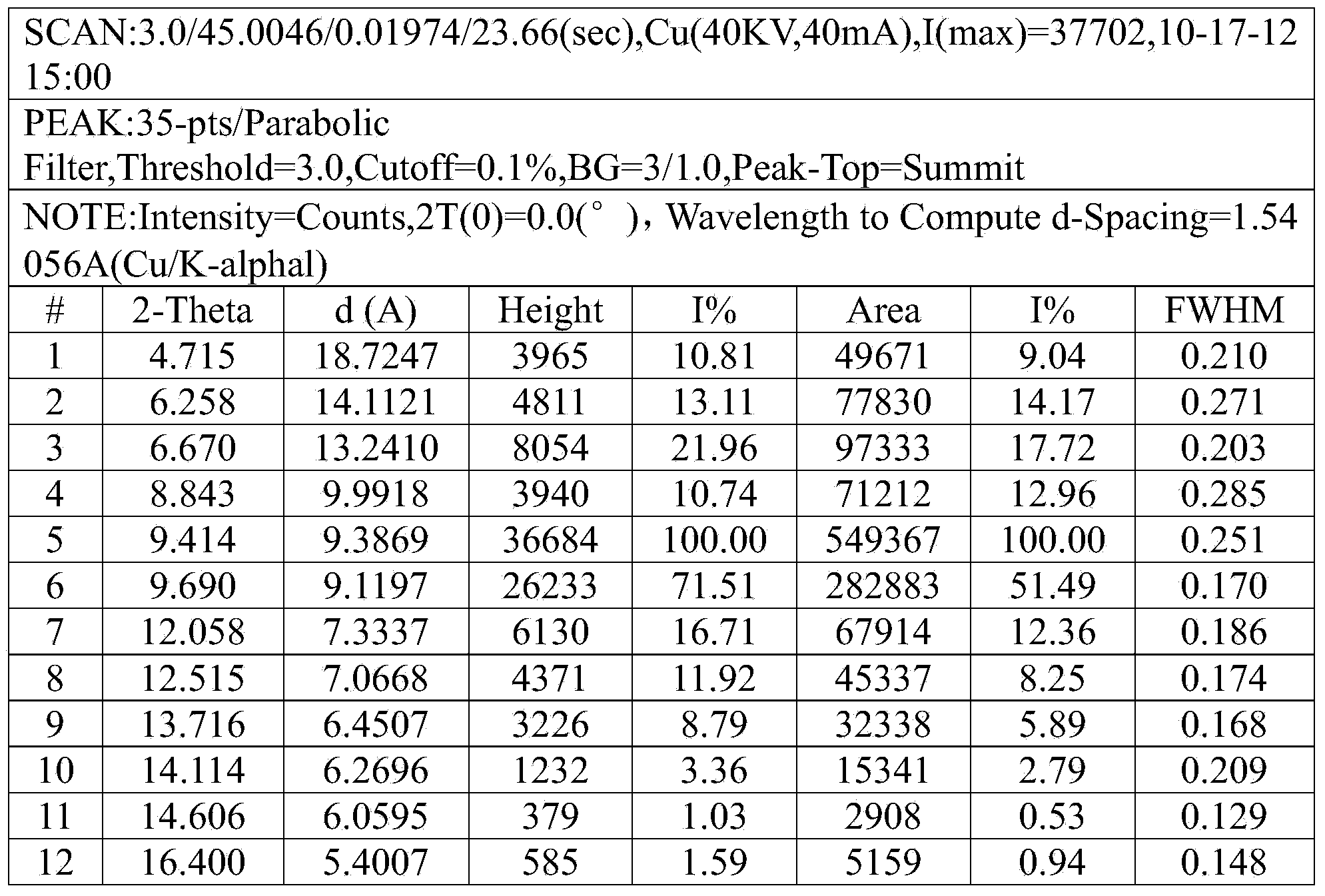
The invention discloses a ceftizoxime sodium compound crystal form. By adopting X-ray powder diffraction determination, the crystal form has the following main characteristic peaks expressed by the diffraction angles of 2 theta + / - 0.2 DEG in a spectrogram: 4.7 DEG, 6.3 DEG, 6.7 DEG, 8.8 DEG, 9.4 DEG, 9.7 DEG, 12.1 DEG, 12.5 DEG, 13.7 DEG, 14.1 DEG, 14.6 DEG, 16.4 DEG, 17.0 DEG, 17.7 DEG, 18.0 DEG, 18.5 DEG, 18.9 DEG, 19.8 DEG, 20.1 DEG, and 21.2 DEG. The invention also discloses a preparation method and a pharmaceutical preparation of the crystal form. Through in-depth study of ceftizoxime sodium, the new ceftizoxime sodium crystal form is obtained after recrystallization, the compound crystal form is obviously of a small granular shape, has uniform particle size distribution and good fluidity, and has the hygroscopicity decreased; and during the production process, no smashing is required, no static electricity is generated, and the stability of the product installed capacity is improved.
Owner:ZHEJIANG YATAI PHARMA
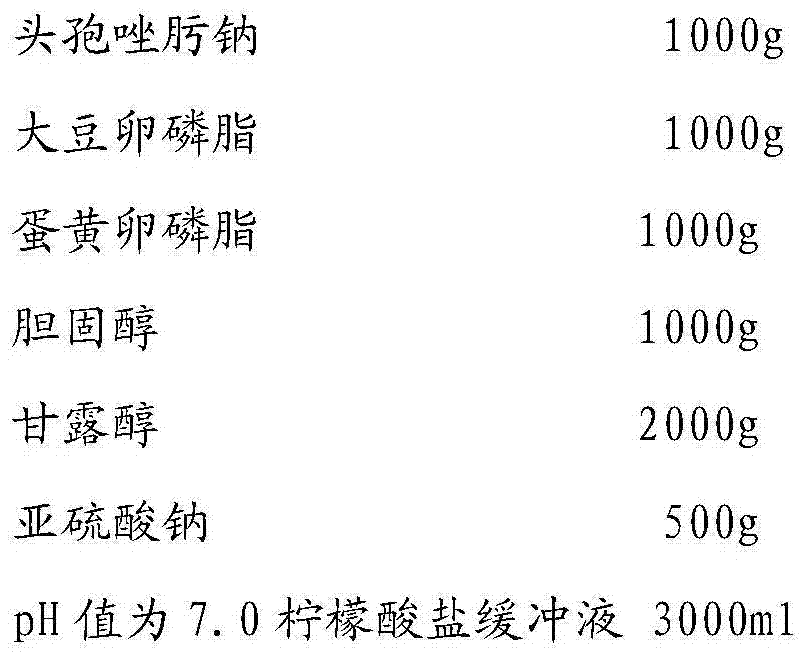
Ceftizoxime sodium liposome injection
InactiveCN101780052ALow priceEasy to operateAntibacterial agentsOrganic active ingredientsFreeze-dryingAntioxidant
The invention relates to ceftizoxime sodium liposome injection and a preparation method therof. The ceftizoxime sodium liposome freezing-drying preparation comprises ceftizoxime sodium, buffer salt system, liposome matrix, freezing-drying excipient and antioxidant. By selecting particular buffer salt system and the liposome matrix, the ceftizoxime sodium liposome injection has good formation and parcel rate as well as good stability.
Owner:HAINAN LINGKANG PHARMA CO LTD
Ceftizoxime sodium for injection and preparation method thereof as well as synthetic method for ceftizoxime sodium serving as crude drug
InactiveCN102718779AHigh purityNo pollution in the processOrganic chemistryCLARITYCeftizoxime Sodium
The invention provides ceftizoxime sodium for injection and a preparation method thereof as well as a synthetic method for ceftizoxime sodium serving as a crude drug. The ceftizoxime sodium for injection has the advantages of moderate grain size range, high purity, low impurity content, high and stable quality, good clarity and the like, so that the sub-packaging efficiency during production is high, the difference of the packaging amount is small, the dissolution speed of drugs in the clinical application is high and the dissolubility is good. The preparation method of the ceftizoxime sodium for injection is simple in process, low in energy consumption, low requirement on equipment and low in cost, is suitable for large-scale industrial production and application and has wide application prospect. The synthetic method for ceftizoxime sodium serving as crude drug is simple in process, low in energy consumption, low requirement on the equipment and low in cost, and is suitable for large-scale industrial production and application.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Preparation method of high purity Ceftizoxime
The invention relates to a preparation method of high purity Ceftizoxime. The method comprises the following steps of: (1) conducting adsorption on Ceftizoxime with a strong-acid ion exchange resin, then carrying out elution, collecting the elution solution, and performing pressure reduced concentration so as to obtain Ceftizoxime acid; (2) performing neutralization with an alkali sodium salt aqueous solution, and implementing filtration to remove insoluble matters to obtain a Ceftizoxime aqueous solution; and (3) adding a proper amount of ethanol into the aqueous solution, controlling the temperature and carrying out recrystallization, thus obtaining high purity Ceftizoxime.
Owner:SUZHOU ERYE PHARMA CO LTD
Method for preparing ceftizoxime powder injection for injection and use thereof
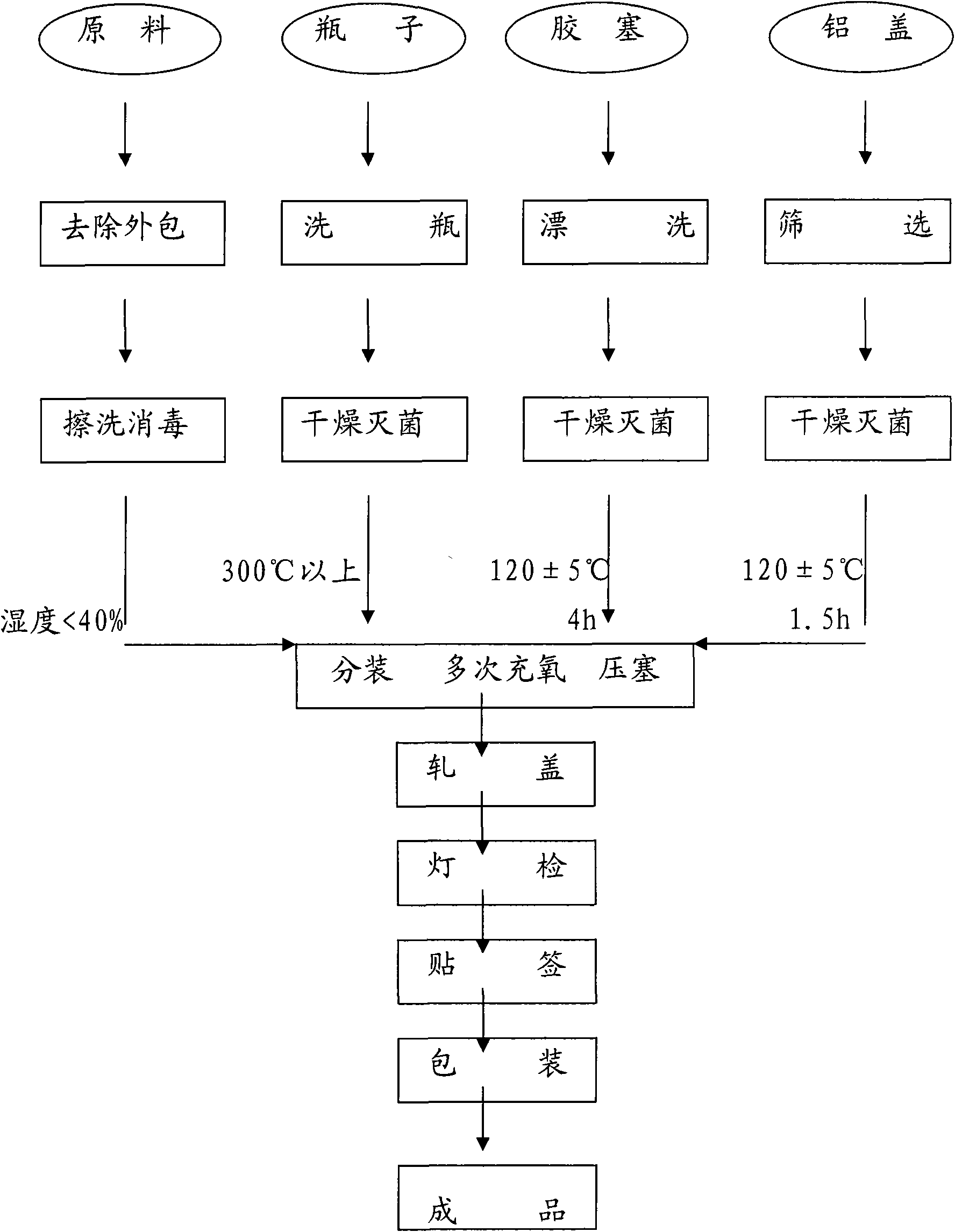
InactiveCN101565105ALow oxygenImprove stabilityAntibacterial agentsOrganic active ingredientsDecompositionNitrogen
The invention provides a method for preparing ceftizoxime powder injection for injection and use thereof. The method for preparing ceftizoxime powder injection for injection provided by the present invention includes steps of filling ceftizoxime raw material powder into bottle, filling nitrogen, fastening plug and rolling cover, and the method is carried out under condition that humidity is less than 40%, and the nitrogen filling operation is carried out under multistage nitrogen-filling protection. Because environmental relative humidity is successfully controlled in the preparation process, preparation humidity is less than 40%, product stability is insured, and by employing multistage nitrogen-filling protection, problem of easy oxidation and decomposition of the product is solved, and expiry date is prolonged. The ceftizoxime powder injection prepared by the method has moisture content less than 8.0%. The method for preparing ceftizoxime powder injection can stability control preparation humidity, reduce oxygen content in finished product, and increase stability of the finished product, and safety of packaged clinic medicament.
Owner:HAIKOU PHARMA FACTORY
Ceftizoxime sodium preparation and refining method
The invention relates to a ceftizoxime sodium preparation and refining method through utilizing ceftizoxime acid. The method comprises the following steps: producing product-ceftizoxime sodium through utilizing the ceftizoxime acid by the action of a salt forming agent; achieving the purposes of refining and purifying through recrystallization; and finally obtaining high purityceftizoxime sodium. According to the invention, the product quality of API is improved, the toxic side effect is reduced, and safety is ensured during preparation of anti-bacterial medicines. Compared with the prior art, the method has the advantages of high product purity, simplicity and convenience in operation, low cost, high yield, reduced toxic side effect, and suitability for industrialized production.
Owner:TIANJIN GREENPINE PHARMA
Ceftizoxime sodium novel crystal form capable of reducing anaphylactic reactions and preparation thereof
ActiveCN105622635ALow impurity contentLow residual organic solventsAntibacterial agentsOrganic active ingredientsChemical compoundX-ray
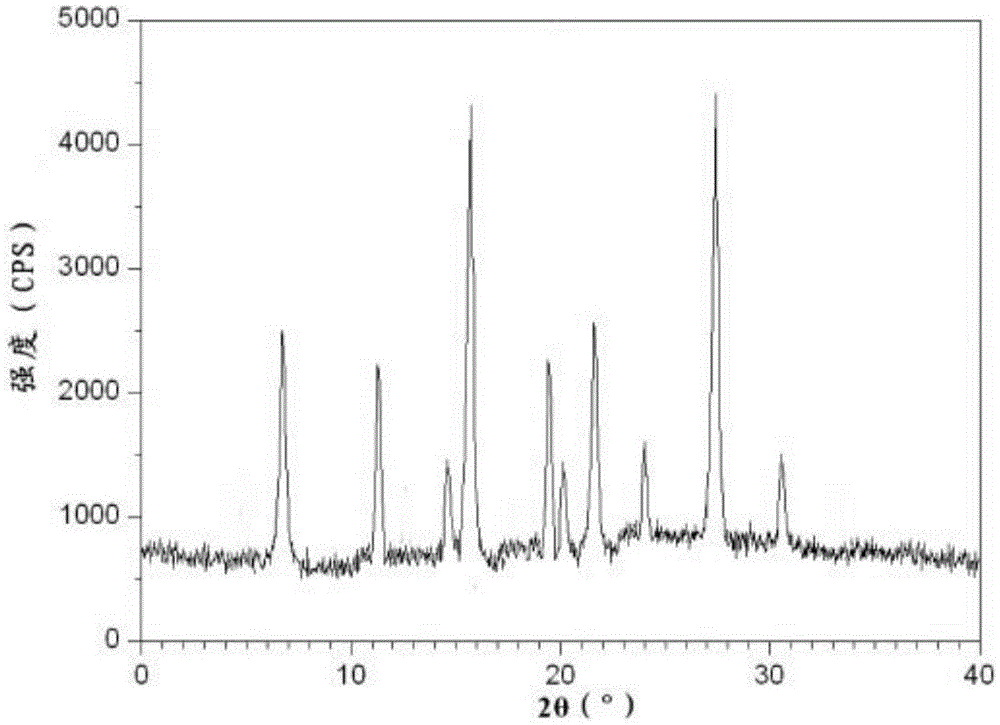
The invention discloses a ceftizoxime sodium novel crystal form capable of reducing anaphylactic reactions, a preparation method and a pharmaceutic preparation of the ceftizoxime sodium novel crystal form. Ceftizoxime sodium is measured through an X-ray powder diffraction method, and feature diffraction peaks are displayed at the 6.8-degree position, the 11.5-degree position, the 14.6-degree position, the 15.8-degree position, the 19.4-degree position, the 20.2-degree position, the 21.8-degree position, the 24.0-degree position, the 27.4-degree position and the 30.5-degree position of an X ray powder diffraction pattern expressed by a 2 theta + / -0.2-degree diffraction angle. The content of ceftizoxime sodium compound impurities is obviously reduced compared with the prior art, and the ceftizoxime sodium novel crystal form has the advantages of being good in stability and fluidity, not prone to absorb moisture, high in dissolution speed, good in clinical effect, low in occurrence rate of adverse reactions and very suitable for clinical application.
Owner:福安药业集团庆余堂制药有限公司 +1
Ceftizoxime sodium-containing pharmaceutical composition
ActiveCN102716075AImprove stabilityImprove toleranceAntibacterial agentsOrganic active ingredientsEthylene diamineVitamin C
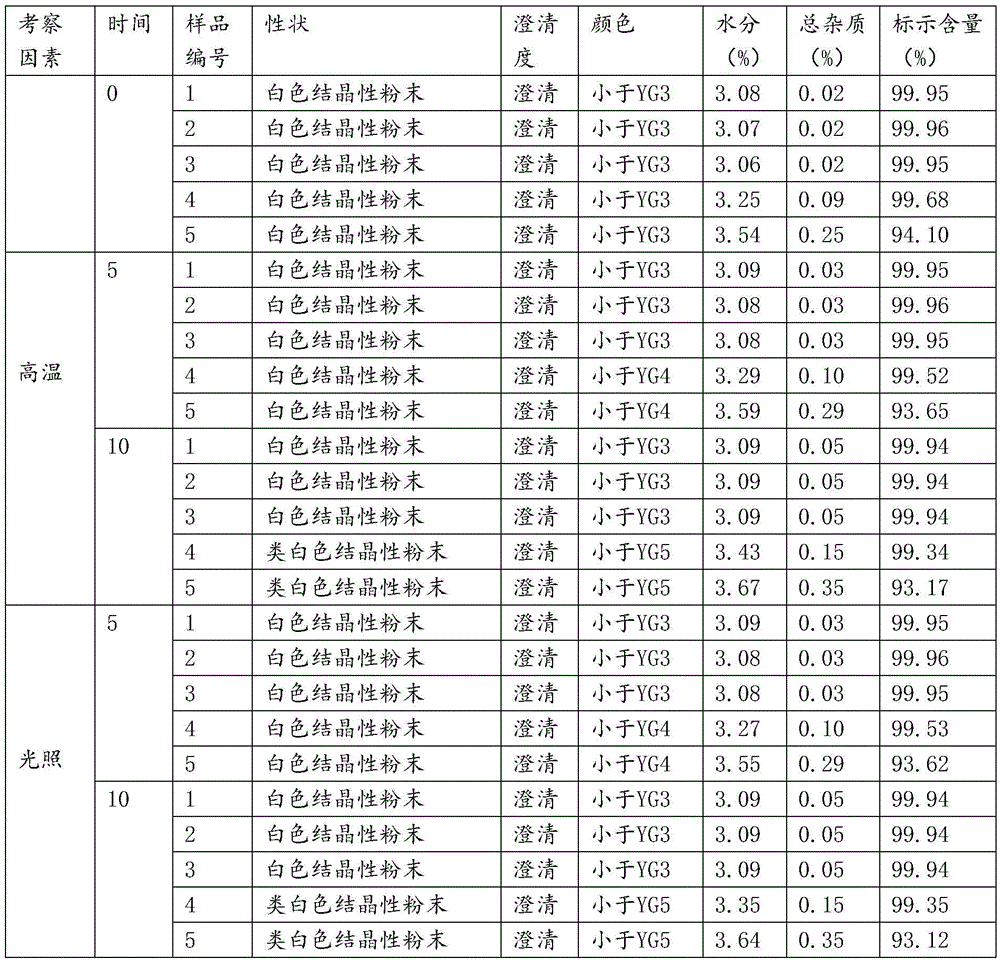
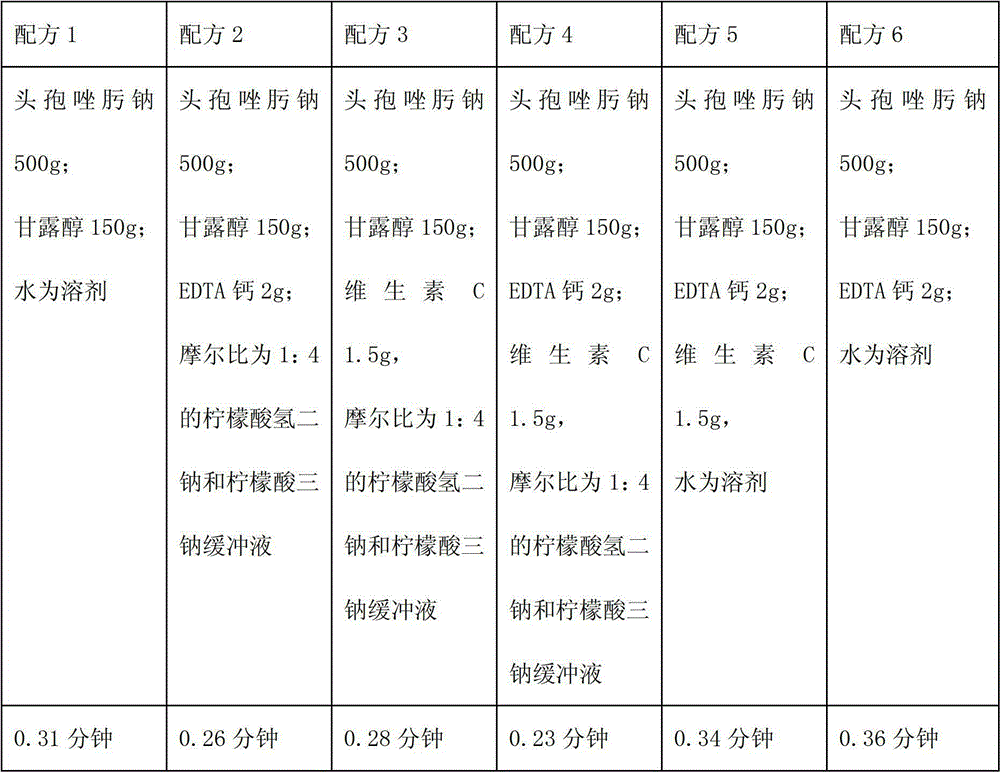
The invention relates to a novel pharmaceutical preparation composition, in particular to a ceftizoxime sodium-containing preparation for injection and a preparation method for the ceftizoxime sodium-containing preparation. The ceftizoxime sodium-containing preparation for injection comprises the following components: ceftizoxime sodium, mannitol, EDTA (Ethylene Diamine Tetraacetic Acid) calcium,vitamin C, sodium hydrogen citrate, 2000ml of trisodium citrate buffer solution and water for injection.
Owner:哈药集团股份有限公司 +1
Method for preparing ceftizoxime
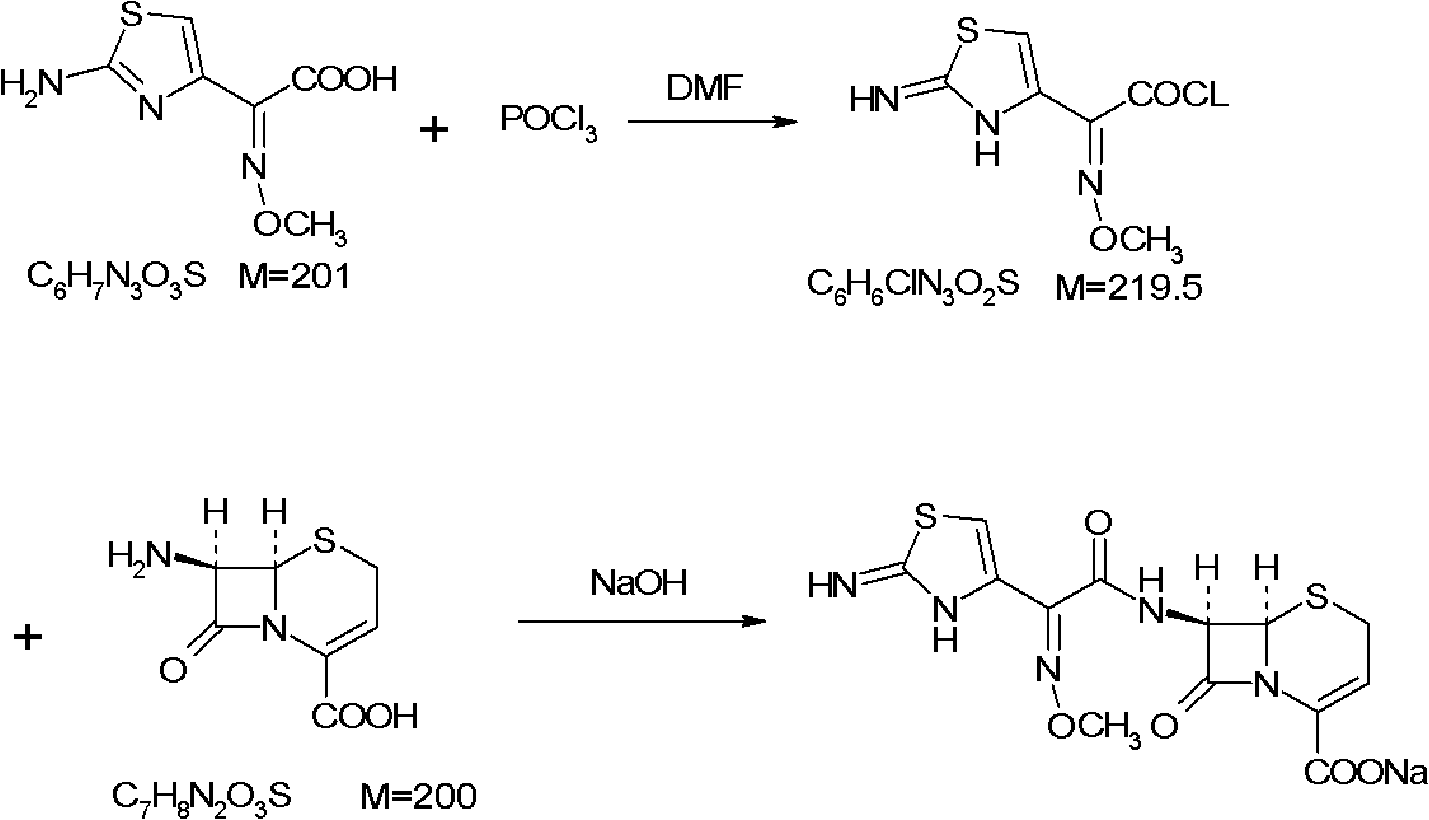
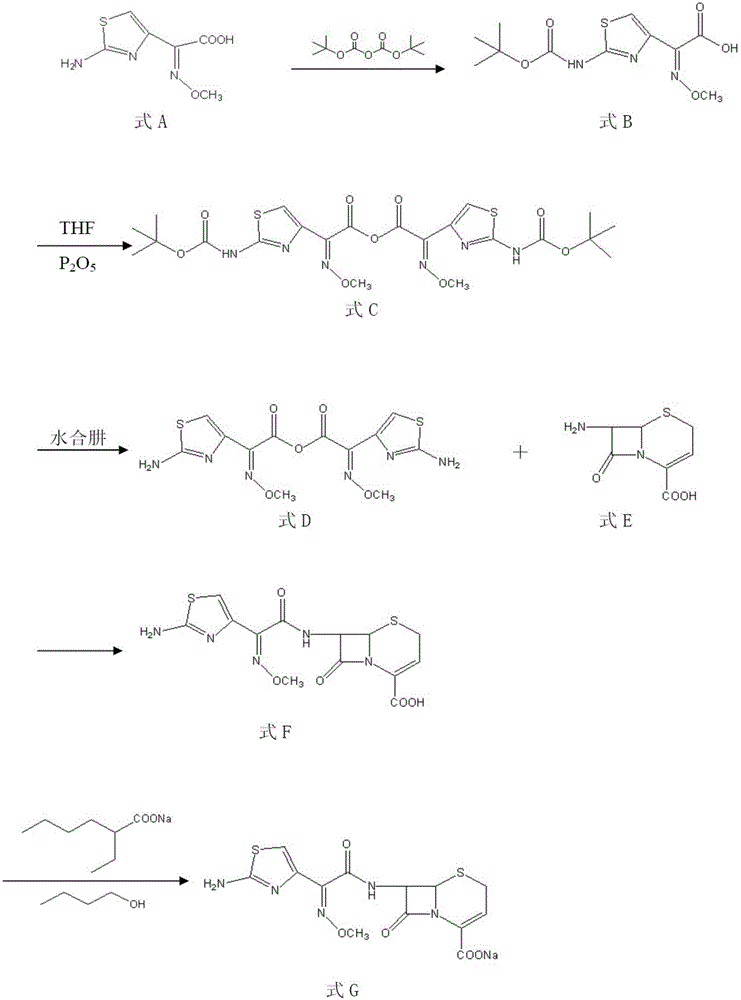
The invention provides a method for preparing ceftizoxime. The method comprises the following steps of: (1) dissolving 2-(2-amino-4-thiazolyl)-2-methoxy-imine acetic acid in an organic solvent, and reacting with N-succinimide under the catalysis of dicyclohexyl carbodiimide / dimethylamino pyridine (DCC / DMAP) to obtain active ester; (2) dissolving the active ester in dichloromethane, and adding 7-amino-3-demethylation-3-cephalosporanic acid (7-ANCA) and alkali for reacting to obtain ceftizoxime acid; and (3) reacting the ceftizoxime acid with a salifying agent to obtain the ceftizoxime, wherein the salifying agent is sodium acetate, sodium ethoxide, sodium 2-ethylhexanoate, sodium isocaproate, sodium bicarbonate or sodium hydroxide.
Owner:SUZHOU ERYE PHARMA CO LTD
Ceftizoxime sodium compound preparation
An antibacterial compound ceftizoxime sodium is prepared from the ceftizoxime or its physiologically acceptable salt and a beta-lectamase depressant in weight ratio of (2-8):1.
Owner:天津新丰制药有限公司
Refining method of ceftizoxime sodium
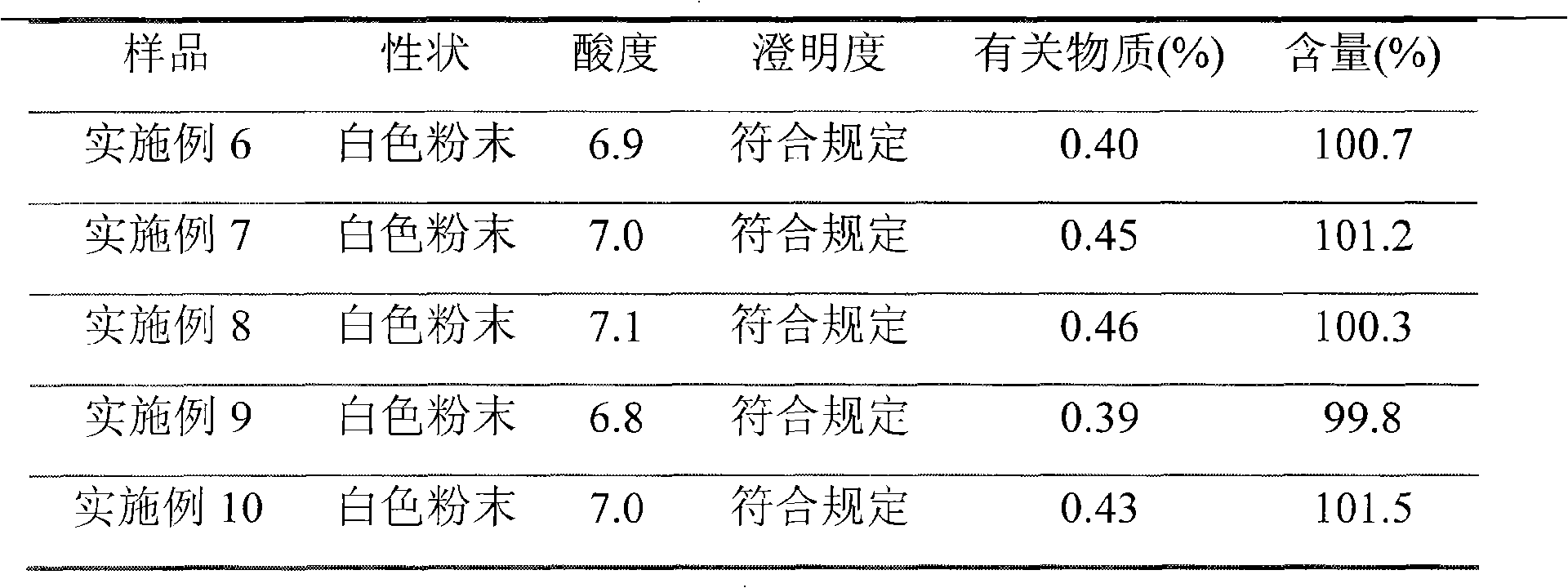
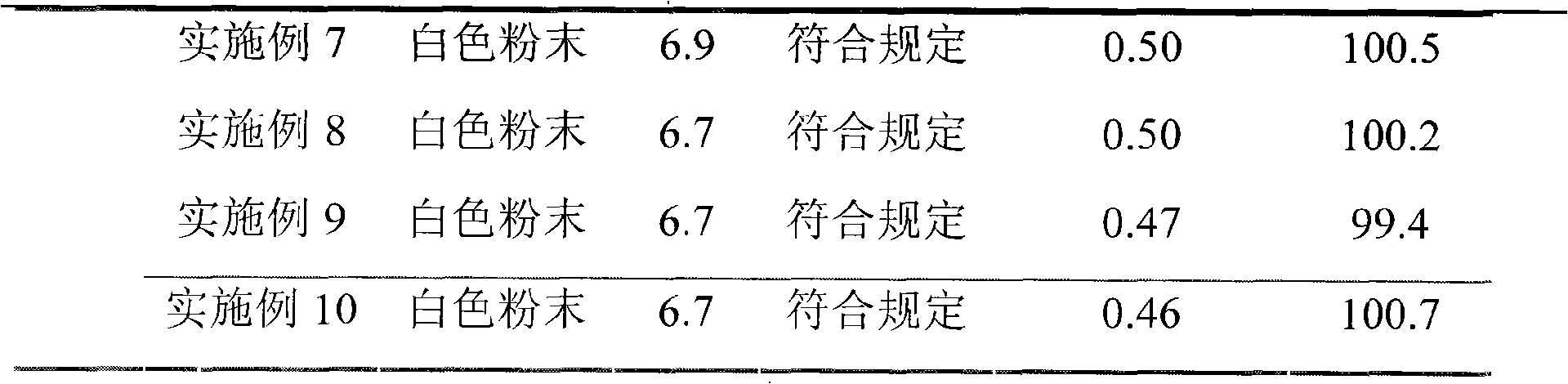
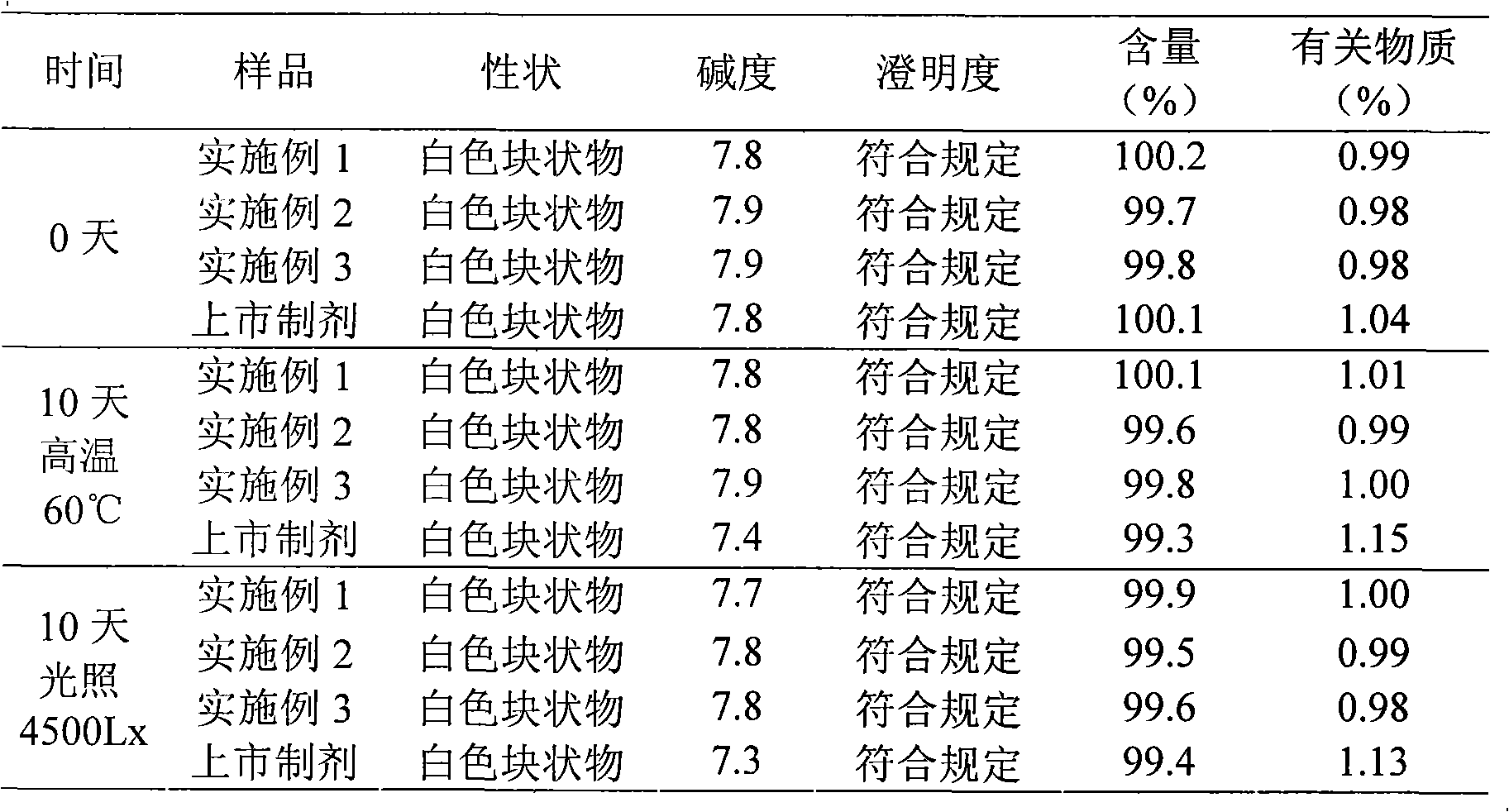
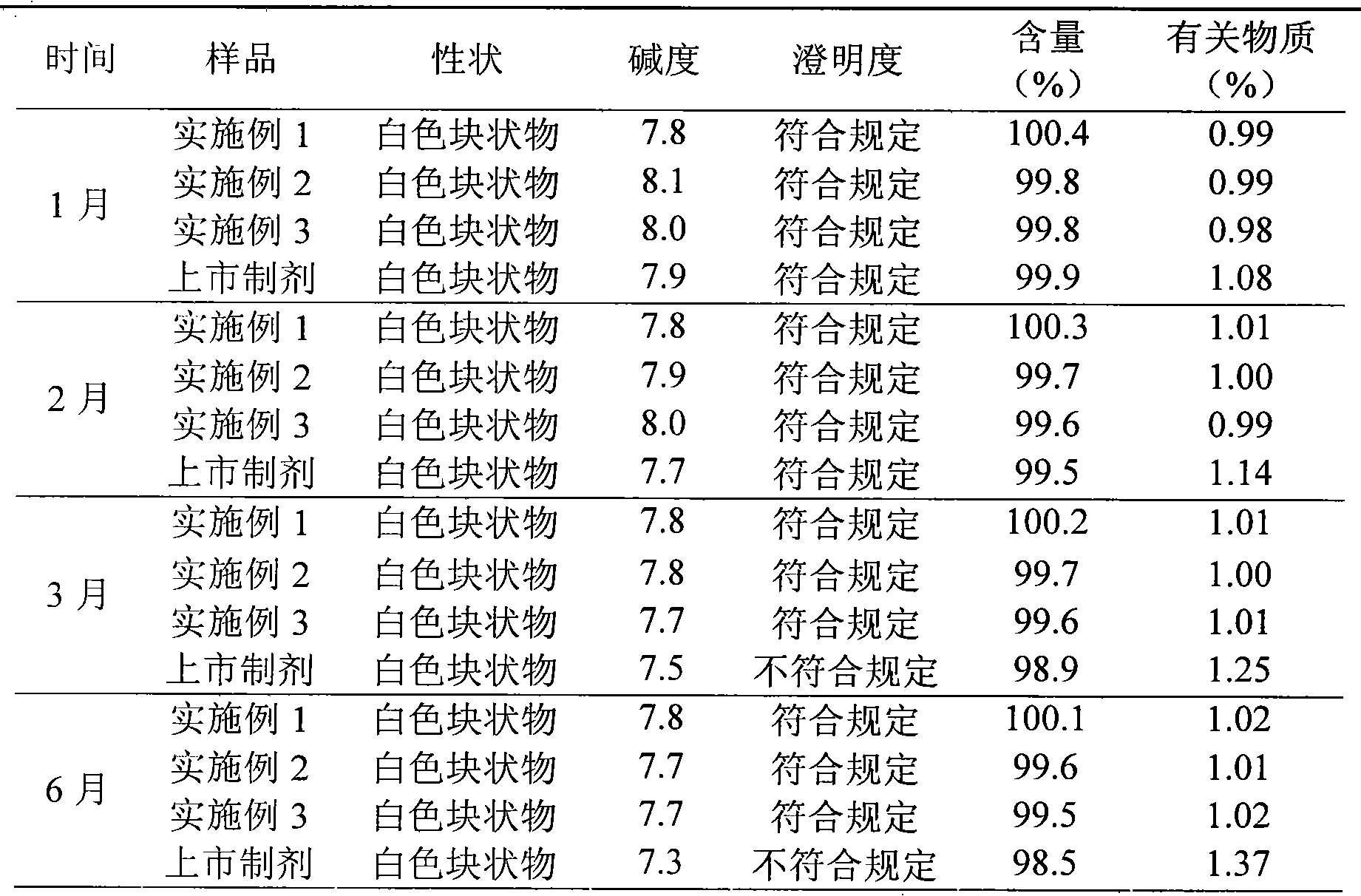
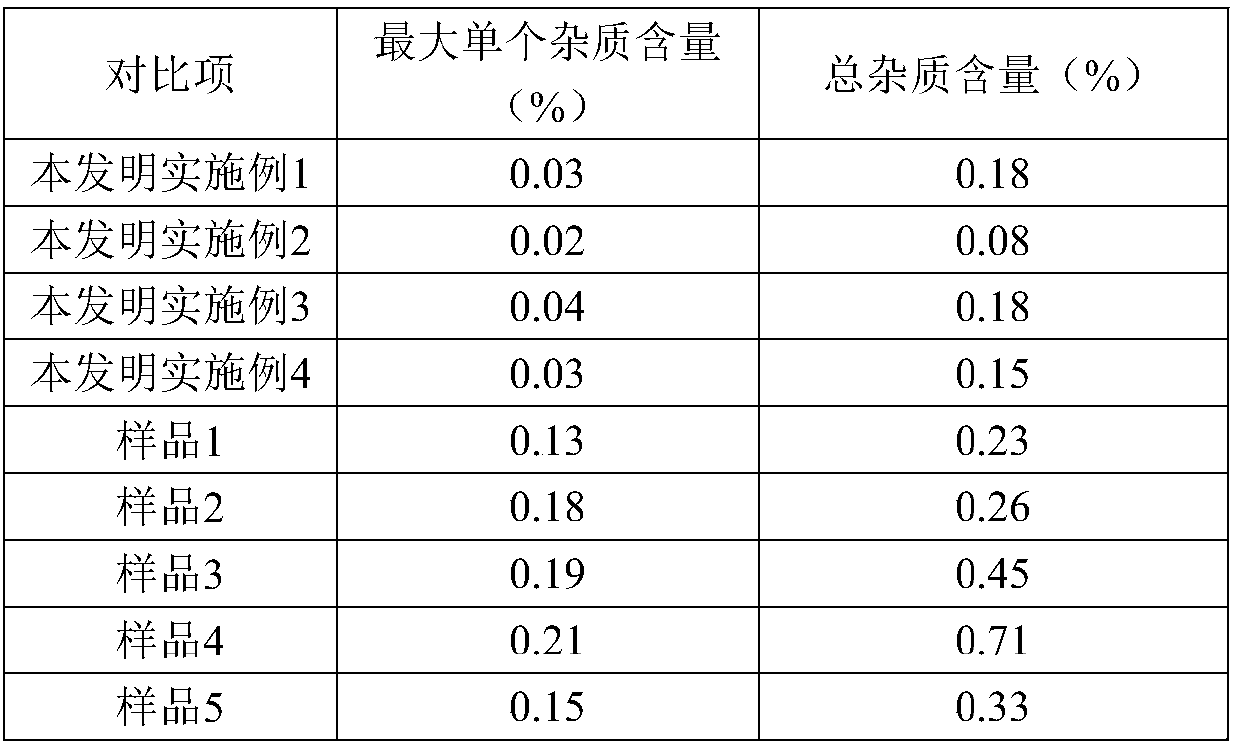
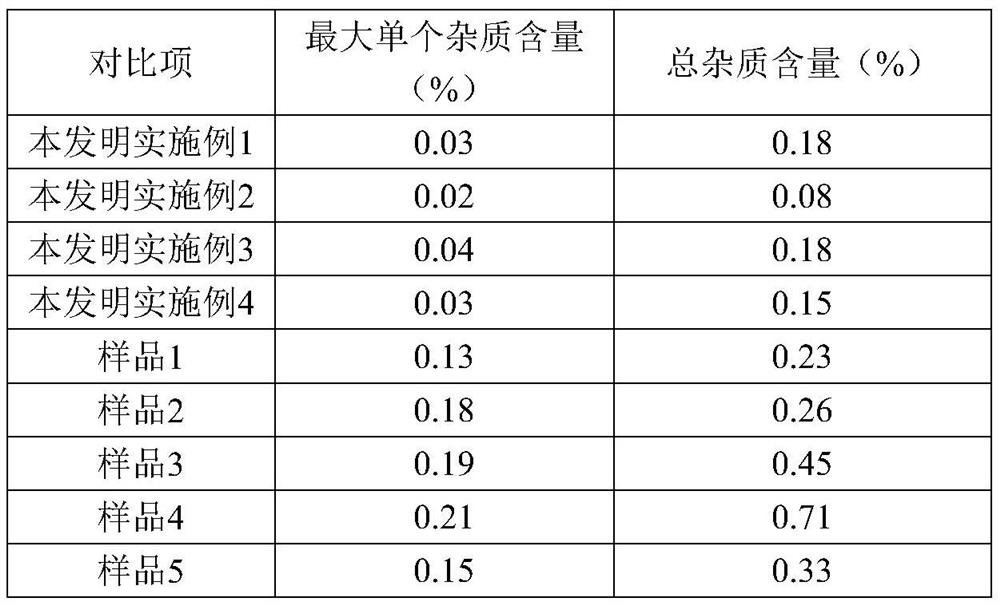
The invention discloses a refining method of ceftizoxime sodium. The refining method comprises the following steps: dissolving cefazoxime acid into water, adding concentrated hydrochloric acid to control the pH value after dissolution and clarification, adding sodium bicarbonate, adding an organic solvent, uniformly carrying out stirring, adding activated carbon for decoloration, carrying out filtering, controlling the temperature to be 15-20 DEG C, rapidly carrying out stirring at frequency of 30-40 HZ, adding acetone, carrying out cooling to 0-5 DEG C, controlling the stirring frequency to be 20 HZ, carrying out crystal growing for 1-2 hours, and carrying out drying to obtain a cefazoxime sodium fine product. According to the refining method disclosed by the invention, the purity of theobtained cefazoxime sodium can reach 99.9% or above, the content of total impurities and the content of a maximum single impurity are respectively controlled to be within 0.2% and 0.05%, the quality of the product is remarkably improved, the refining process is simple and convenient to operate, and the method is suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Children ceftizoxime sodium compound entity and preparation thereof
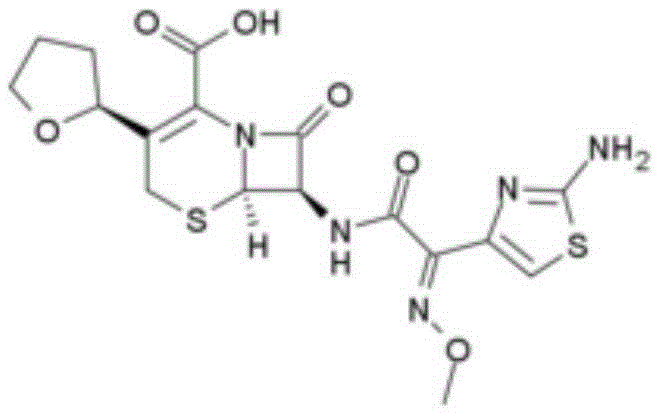
InactiveCN105037390AIncrease internal pressureImprove solubilityPowder deliveryOrganic chemistryTemperature controlStructural formula
The present invention provides a children ceftizoxime sodium compound entity, which has the following structural formula. The preparation steps comprise: (1) dissolving a ceftizoxime sodium crude product in water, carrying out stirring decolorizing with active carbon, and filtering; (2) adding an extraction agent to the filtrate, transferring and filling into a pressure-resistant container, removing gas bubbles, carrying out temperature control freezing, and taking out; and (3) removing the organic phase, adding ethanol in a dropwise manner after the solid melts, stirring at a slow speed, growing the crystal, carrying out filtering washing, and carrying out vacuum drying to obtain the ceftizoxime sodium finished product. According to the present invention, the ceftizoxime sodium prepared by the method has advantages of less impurity, high purity and the like of the ceftizoxime sodium prepared by the conventional process.
Owner:ZHEJIANG CHANGDIAN PHARMA
Cefuroxime sodium compound and new preparation method thereof
InactiveCN102079750BHigh purityIncrease contentAntibacterial agentsOrganic chemistryActivated carbonUpper urinary tract infection
The invention provides a cefuroxime sodium compound and a new preparation method thereof. The method comprises the following steps: performing acid-base reaction, absorbing with activated carbon, and separating and purifying with a chromatographic column. By adopting the method, the purity and content of cefuroxime sodium can be greatly increased, the product quality of the preparation can be increased, the toxic or side effects can be reduced and the safety of the preparations of the medicines for curing respiratory tract infection and urinary tract infection can be ensured; and compared with the prior art, the method has the advantages of simple and practical technology, mild reaction conditions, low cost, high yield and high product purity and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
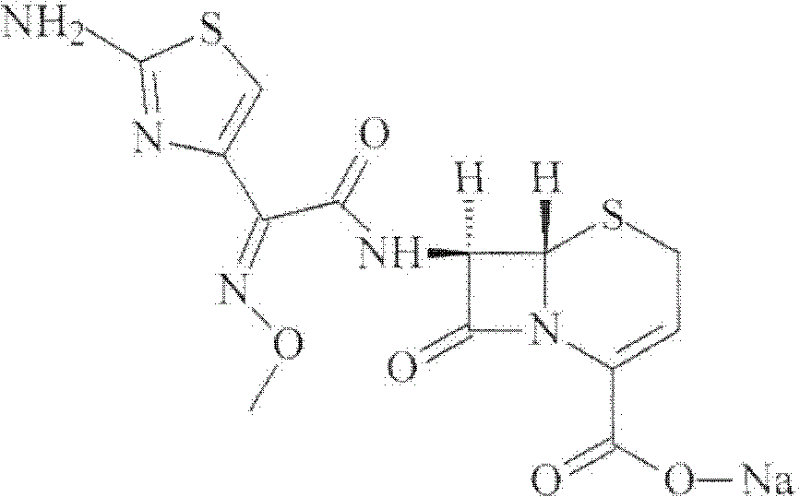
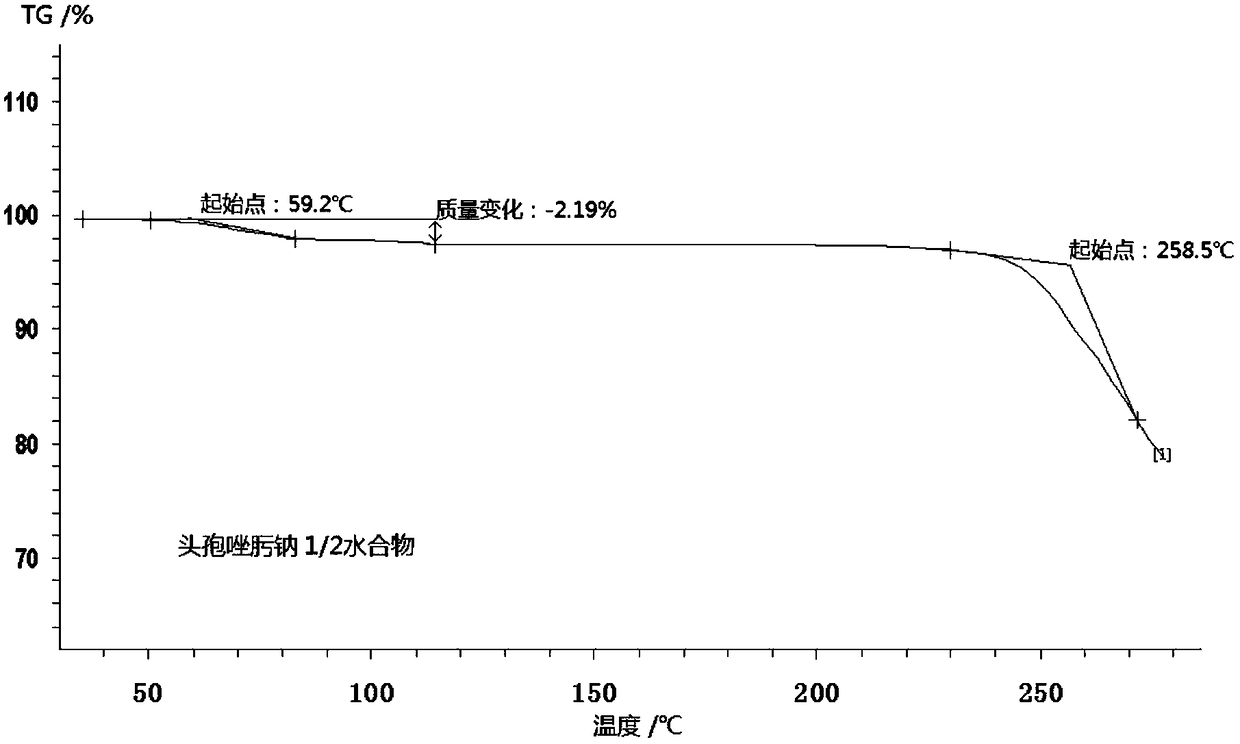
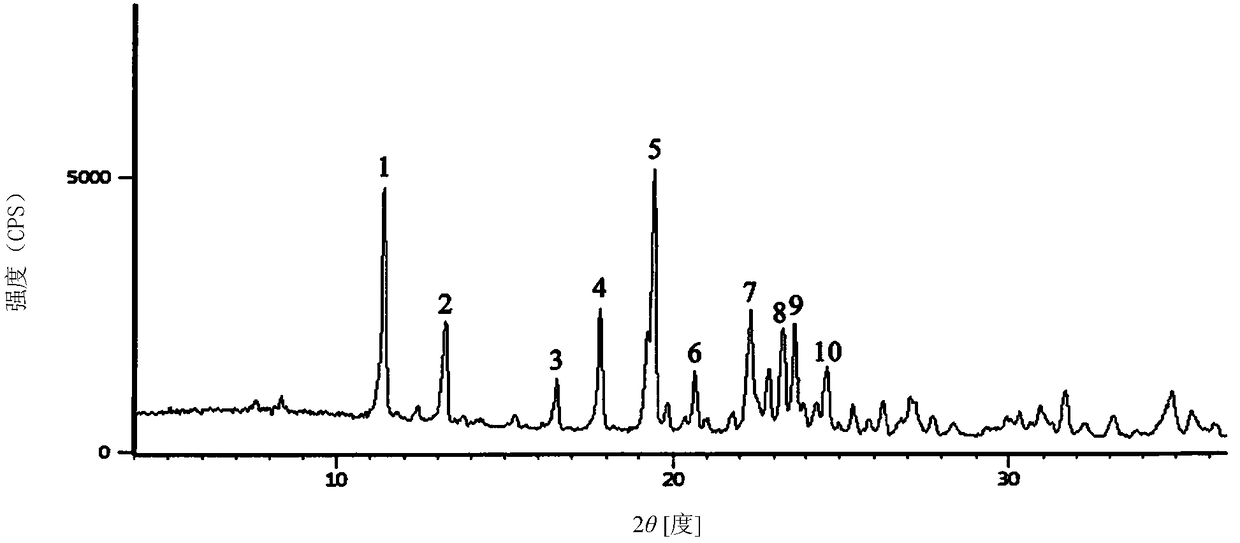
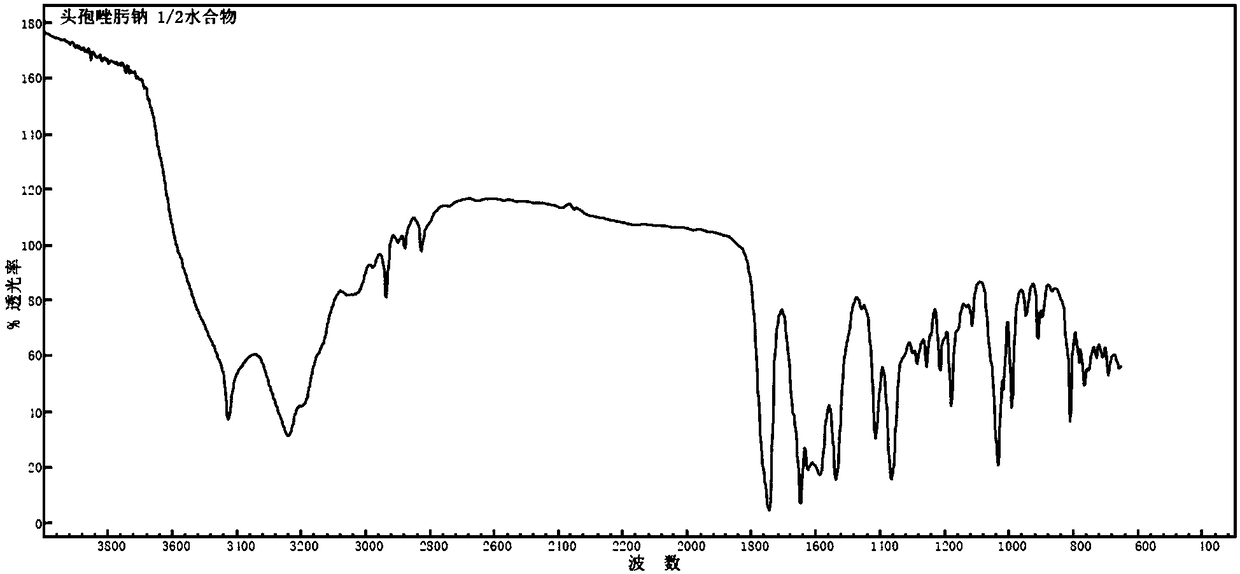
1/2 water ceftizoxime sodium compound
InactiveCN109160921AComplianceHigh yieldAntibacterial agentsOrganic active ingredientsCeftizoxime SodiumRaw material
The invention discloses a 1 / 2 water ceftizoxime sodium compound and a preparation method of the 1 / 2 water ceftizoxime sodium compound, each molar of ceftizoxime sodium contains 1 / 2 molar of water, theceftizoxime sodium compound is white crystalline powder, and the ceftizoxime sodium compound has a characteristic diffraction peak when the angle 2theta of the corresponding main X-ray characteristicdiffraction peak is 11.43 plus or minus 0.2 degrees, 13.24 plus or minus 0.2 degrees, 16.54 plus or minus 0.2 degrees, 17.85 plus or minus 0.2 degrees, 19.46 plus or minus 0.2 degrees, 20.66 plus orminus 0.2 degrees, 22.33 plus or minus 0.2 degrees, 23.30 plus or minus 0.2 degrees, 23.65 plus or minus 0.2 degrees, 24.60 plus or minus 0.2 degrees. The 1 / 2 water ceftizoxime sodium compound prepared through the method is excellent stability and meets the requirement of the raw material of a preparation.
Owner:王秀香
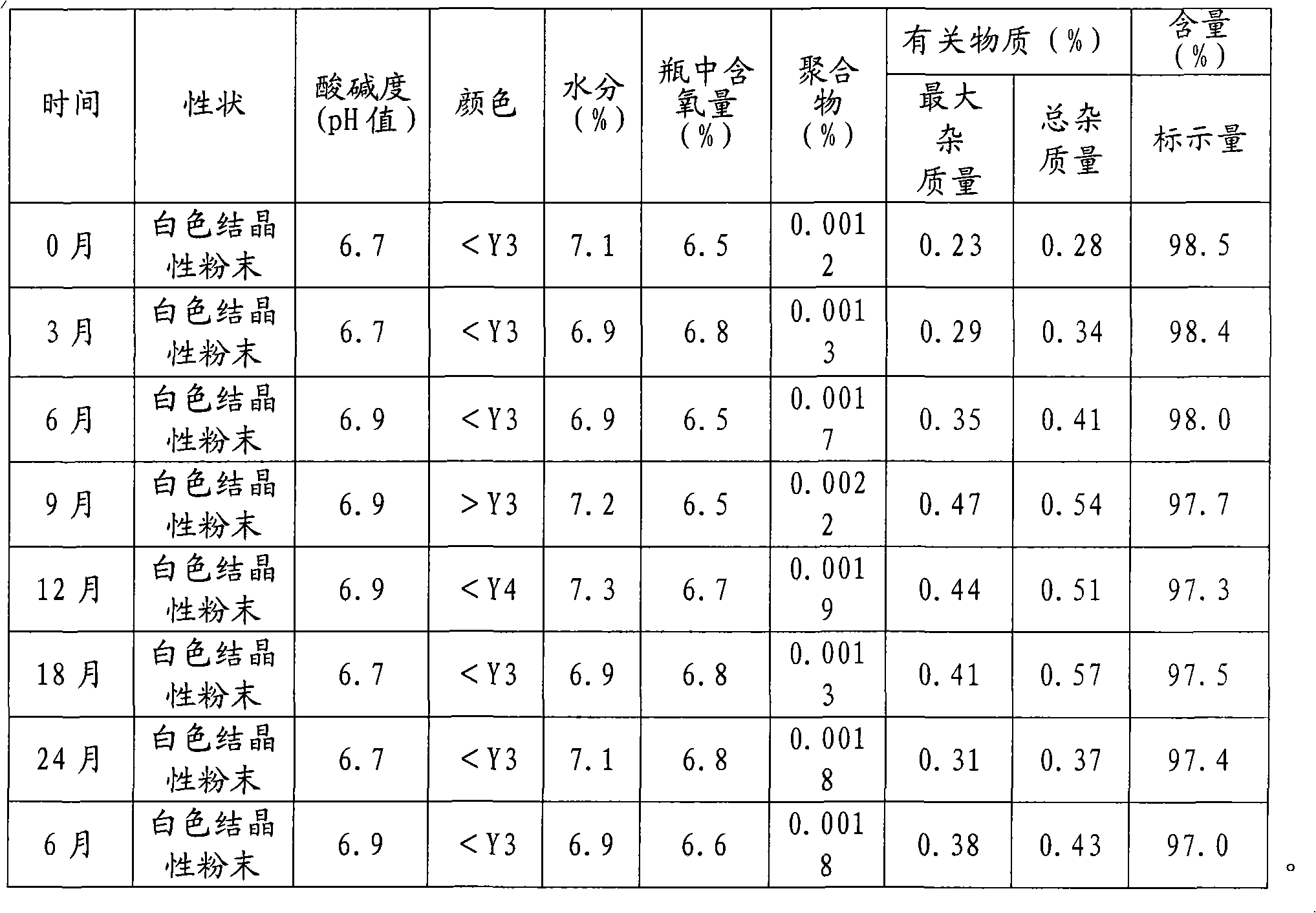
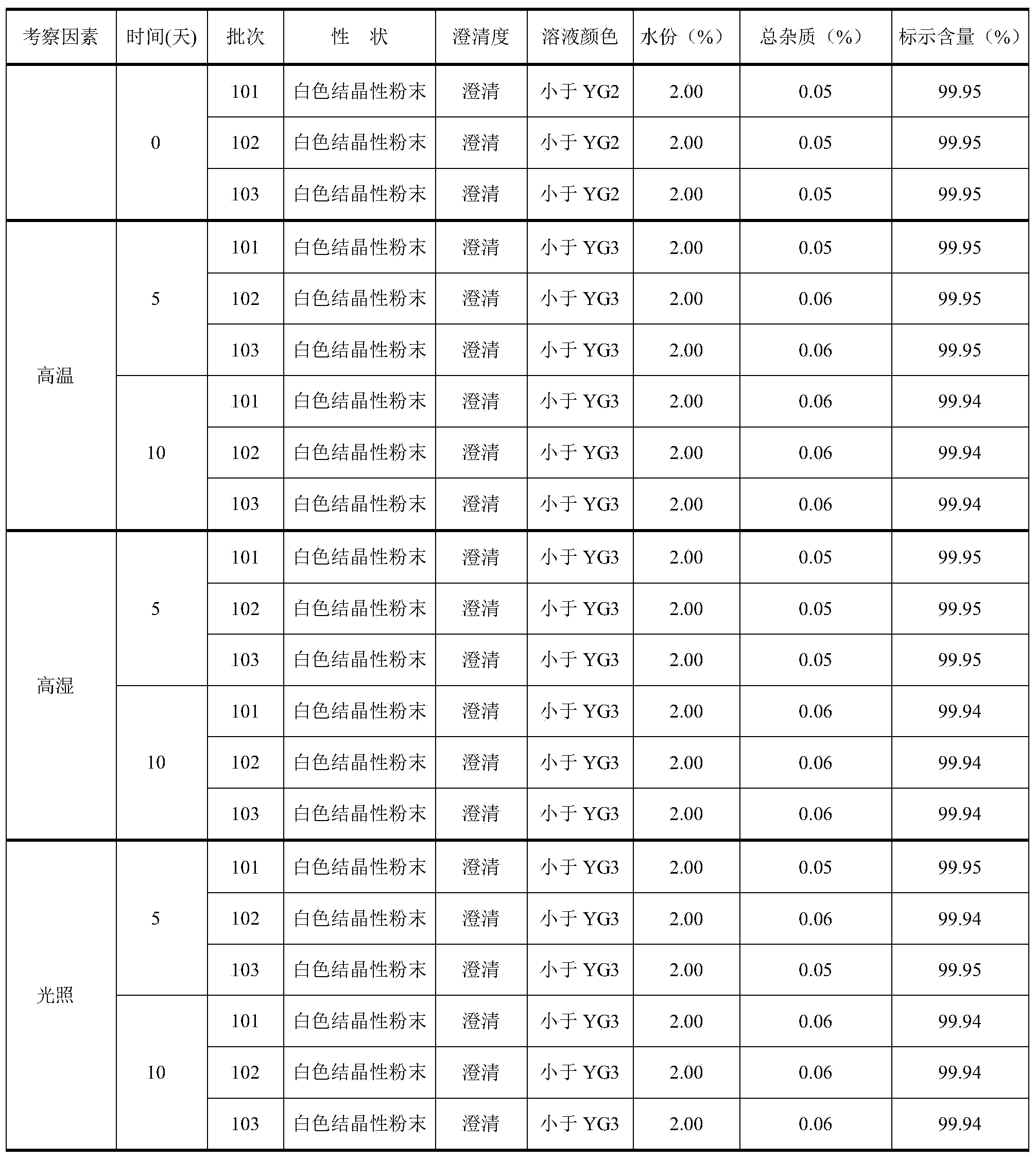
Detection method and production process for ceftizoxime sodium preparation
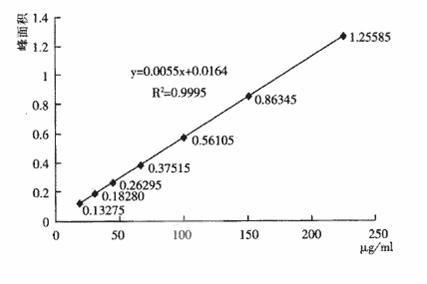
InactiveCN102351883AEasy to separateOrganic chemistryComponent separationCeftizoxime SodiumProcess conditions
The invention discloses a preparation method and a detection method for ceftizoxime sodium. Various factors which influence the quality and yield of the product in the process of producing the ceftizoxime sodium are subjected to experimental analysis and research, and optimal process conditions for producing the ceftizoxime sodium are obtained; and a method which meets the requirement of a national medicine inspection sample and is used for measuring ceftizoxime sodium high polymer is established.
Owner:SUZHOU ERYE PHARMA CO LTD
Ceftizoxime sodium ultrafine powder preparation and preparation method thereof
InactiveCN104042563AHigh purityGood for mass productionAntibacterial agentsPowder deliveryMetallurgyPharmaceutical drug
The invention discloses a ceftizoxime sodium ultrafine powder preparation which comprises 99.0-99.9 weight percent of ceftizoxime sodium and 0.10-1.00 weight percent of sodium carbonate, wherein the granularity of the ceftizoxime sodium ultrafine powder preparation is 1.0-3.0mu m. The invention also discloses a method for preparing the ceftizoxime sodium ultrafine powder preparation. The prepared ceftizoxime sodium ultrafine powder preparation is high in purity, the granularity of powder particles can be controlled to be in a range from 1.0 to 3.0mu m by adjusting the preparation conditions, the powder is in micron-scale, uniform in size distribution and high in re-dissolubility, and active ingredients in the medicine are absorbed, so that the curative effect of the medicine is enhanced. The preparation method is mild in reaction conditions, simple in steps and process and easy to regulate, and volume production of the ceftizoxime sodium ultrafine powder preparation can be conveniently realized.
Owner:杭州长典老一元健康管理有限公司
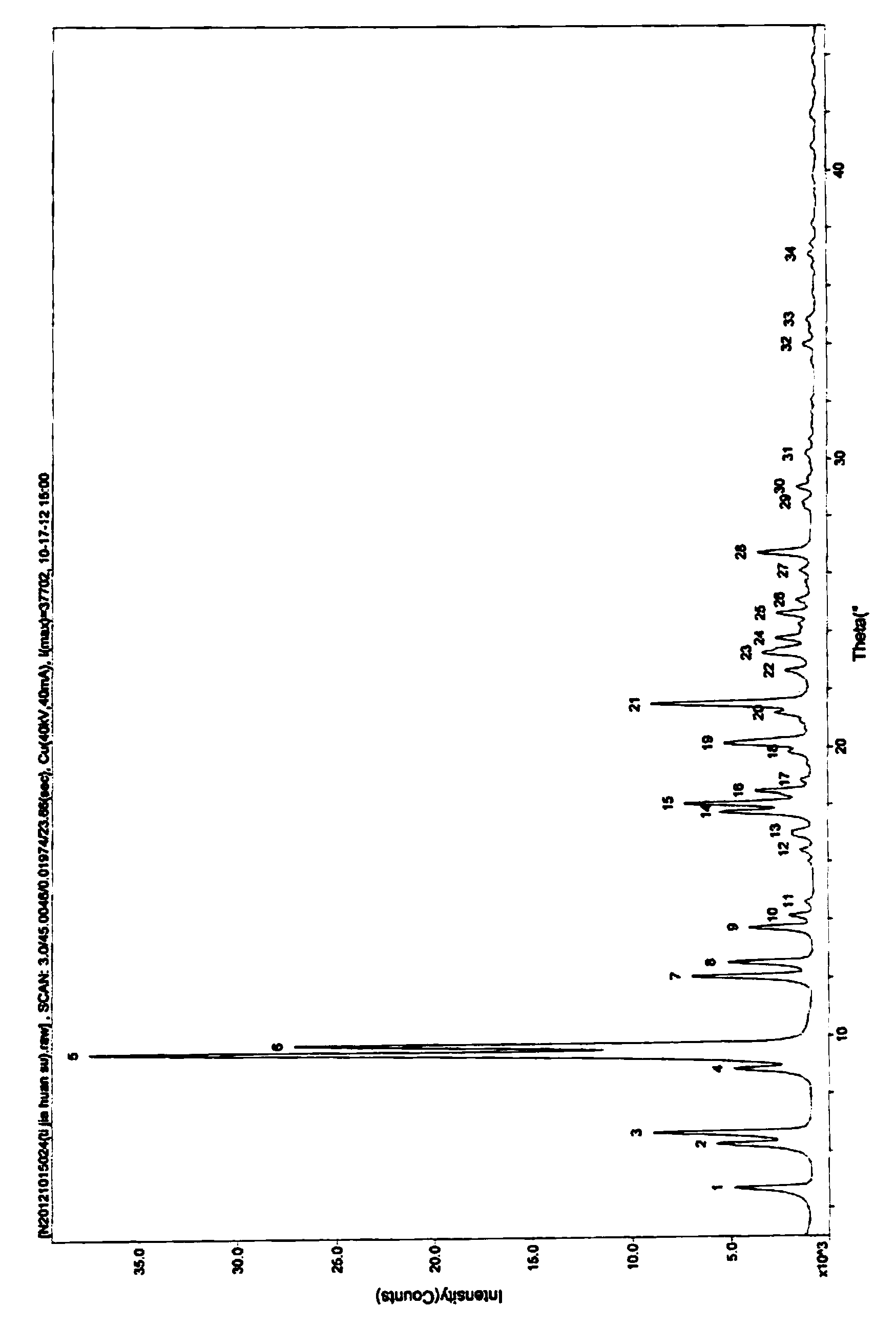
Ceftizoxime sodium compound
The invention relates to the field of compounds, and in particular relates to a ceftizoxime sodium compound. The X-ray powder diffraction diagram of the ceftizoxime sodium compound obtained by Cu-K alpha-ray measurement is shown in figure 1, the main particle size of the ceftizoxime sodium compound is 220-280mu m, and the distribution width is 120-390mu m. The ceftizoxime sodium compound provided by the invention has good stability and liquidity, and is not easy for moisture absorption, high in dissolution rate, good in clinical effect, low in incidence of adverse reactions, and very suitable for clinical application.
Owner:YOUCARE PHARMA GROUP
Ceftizoxime sodium liposome composition for injection and preparation method thereof
ActiveCN104490785AHigh parcel rateReduce leak rateAntibacterial agentsOrganic active ingredientsMass ratioCholesterol
The invention discloses a ceftizoxime sodium liposome composition for injection and a preparation method thereof. The ceftizoxime sodium liposome composition comprises ceftizoxime sodium, phospholipid, cholesterol and citrate buffer solution, wherein the mass ratio of the phospholipid to cholesterol is (1.5-2):1. The ceftizoxime sodium liposome composition provided by the invention has the advantages of good stability, high encapsulation efficiency, low leakage rate, and is suitable for industrial production.
Owner:FUAN PHARM (GRP) CO LTD +1
A kind of refining method of ceftizoxime sodium
The invention discloses a method for refining ceftizoxime sodium. The refining steps include: adding water to dissolve ceftizoxime acid, adding concentrated hydrochloric acid to control pH after dissolving, adding sodium bicarbonate, adding an organic solvent, stirring evenly, and adding activated carbon Perform decolorization, filter, control the temperature at 15-20°C, stir rapidly at 30-40HZ, add acetone, cool down to 0-5°C, control the stirring speed at 20HZ, carry out crystal growth for 1-2h, and dry to obtain ceftizole Oxime sodium boutique. Through the refining method of the present invention, the purity of the obtained ceftizoxime sodium can reach more than 99.9%, the total impurities and the largest single impurity are respectively controlled within 0.2%, 0.05%, the quality of the product has been significantly improved, and the refining process is easy to operate , suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
Preparation method of high purity Ceftizoxime
Owner:SUZHOU ERYE PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com