Detection method and production process for ceftizoxime sodium preparation
A technology of ceftizoxime sodium and ceftizoxime acid, which is applied in the field of medicine and can solve problems such as uncollected
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
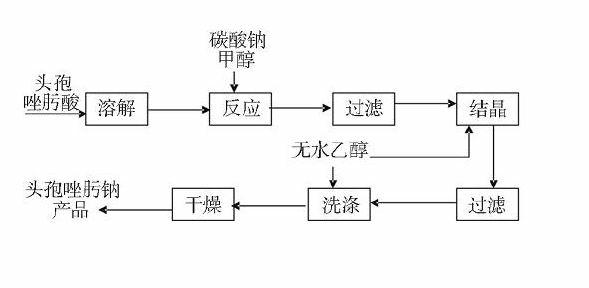
[0055] 1. The process conditions for preparing ceftizoxime sodium
[0056] Reaction temperature 28°C, anhydrous sodium carbonate: ceftizoxamic acid (mass ratio) = 0.15: 1.0, reaction time 1.5h, control the pH value before crystallization to 6.7, ethanol: ceftizoxamic acid = 30ml: 1.0g, ethanol drops The acceleration is 450ml / h, and the crystal growth time is 2h. The product yield reaches 95.5%, and the product quality meets the Pharmacopoeia standard.
[0057] 2. The process conditions for preparing ceftizoxime sodium
[0058] Reaction temperature 30 ℃, anhydrous sodium carbonate: ceftizoxamic acid (mass ratio) = 0.15: 1.0, reaction time 2h, control pH value before crystallization is 7.0, ethanol: ceftizoxamic acid = 30ml: 1.0g, ethanol drop rate 450ml / h, crystal growth time 2h. The product yield reaches 96%, and the product quality meets the Pharmacopoeia standard.
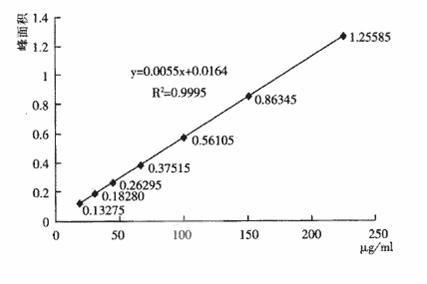
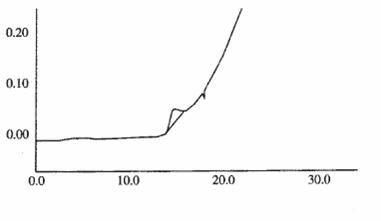
[0059] 3. Inspection of polymers in ceftizoxime sodium injection preparations
[0060] The chromatographi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com