High-purity ceftizoxime sodium and preparation thereof
A technology of ceftizoxime and ceftizoxime acid, which is applied in the field of medicine, can solve the problems affecting the application of ceftizoxime or its salt, large side effects, poor stability, etc., and achieve the effects of low cost, improved stability, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
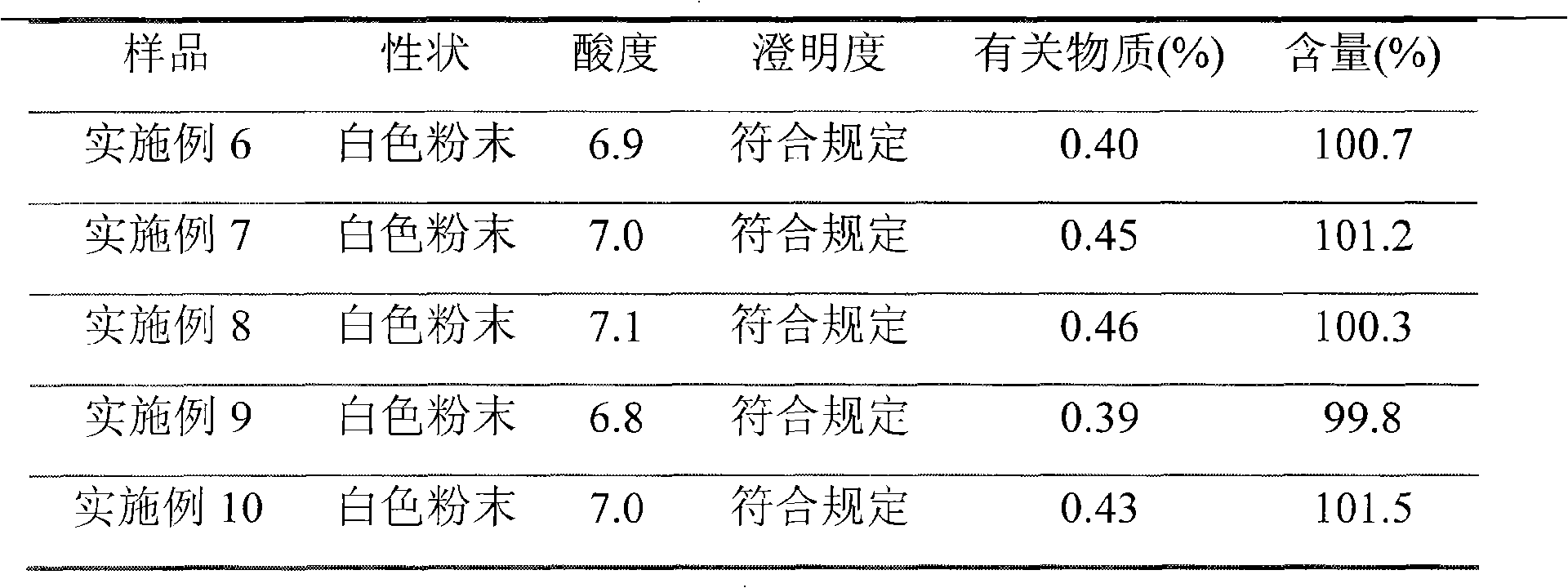
Embodiment 1
[0043] (1) Dissolve 500 g of ceftizoxime sodium crude product in 2 L of purified water, add sulfuric acid to adjust the pH value to 4;
[0044] (2) Extract with 6L n-butanol, separate the organic phase, flow the organic phase through anhydrous magnesium sulfate column for dehydration, then concentrate under reduced pressure to dryness, and get the column product;
[0045] (3) After suspending 20 kg of 800-mesh alumina with activity level II with n-hexane, remove air bubbles, evenly add it to a glass chromatography column with an effective column length of 400 cm and an internal diameter of 20 cm, and concentrate the above-mentioned ceftizole to dryness Add oxime sodium crude product to dissolve in 800ml eluent (cycloheptane and chloroform volume ratio are 1: 7), filter out the insoluble matter, put it on the column head with plunger type solvent pump, then pump into eluent (cycloheptane Alkanes and chloroform (volume ratio is 1: 7) elution, column pressure is 3MPa, collect fra...
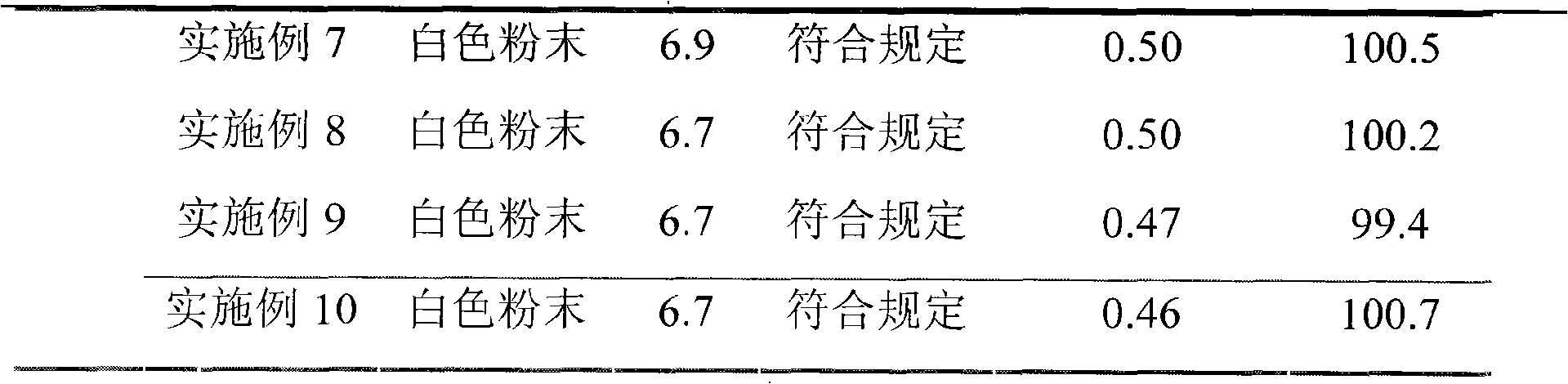
Embodiment 2
[0050] (1) Take 800g of ceftizoxime sodium crude product and dissolve it in 3L of purified water, add phosphoric acid to adjust the pH value to 3;
[0051] (2) Extract with 10L cyclooctane, separate the organic phase, flow the organic phase through an anhydrous calcium chloride column for dehydration, then concentrate under reduced pressure to dryness to obtain a column product;
[0052] (3) After suspending 10 kg of 100-mesh aluminum oxide with activity level II with n-hexane, remove air bubbles, and evenly add it to a glass chromatography column with an effective column length of 200 cm and an internal diameter of 10 cm, and the above-mentioned concentrated to dry ceftizoxime Add 1000ml of eluent (volume ratio of 1-nonene to 1,2-dichloroethane is 1:4) to dissolve the crude sodium product, filter out the insoluble matter, put it on the column head with a plunger solvent pump, and then pump it into the eluent (1-nonene to 1,2-dichloroethane volume ratio is 1:4), the column pre...
Embodiment 3
[0057] (1) Dissolve 600g of ceftizoxime sodium crude product in 2L of purified water, add hydrochloric acid to adjust the pH value to 2.5;
[0058] (2) Extract three times with 5L chloroform, separate the organic phase, flow the organic phase through an anhydrous sodium sulfate column for dehydration, and then concentrate under reduced pressure to dryness to obtain a column product;
[0059] (3) After suspending 5kg of 50-mesh alumina with activity level II with n-hexane, remove air bubbles, and evenly add it to a glass chromatography column with an effective column length of 20cm and an internal diameter of 5cm, and the above-mentioned concentrated to dry ceftizoxime Add 600ml of eluent (normal hexane to dichloromethane volume ratio is 1:10) to dissolve the sodium crude product, filter out the insoluble matter and put it on the column head with a plunger solvent pump, then pump in the eluent (normal hexane and dichloromethane Methane (volume ratio: 1:10) was eluted, column pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com