Ceftizoxime sodium drug injection powder and preparation method thereof, as well as synthetic method of bulk drug ceftizoxime sodium
A technology of cefizoxime sodium and cefizoxime acid is applied in the field of drug synthesis and preparation, and can solve the problems of complex chromatography column method, difficult operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
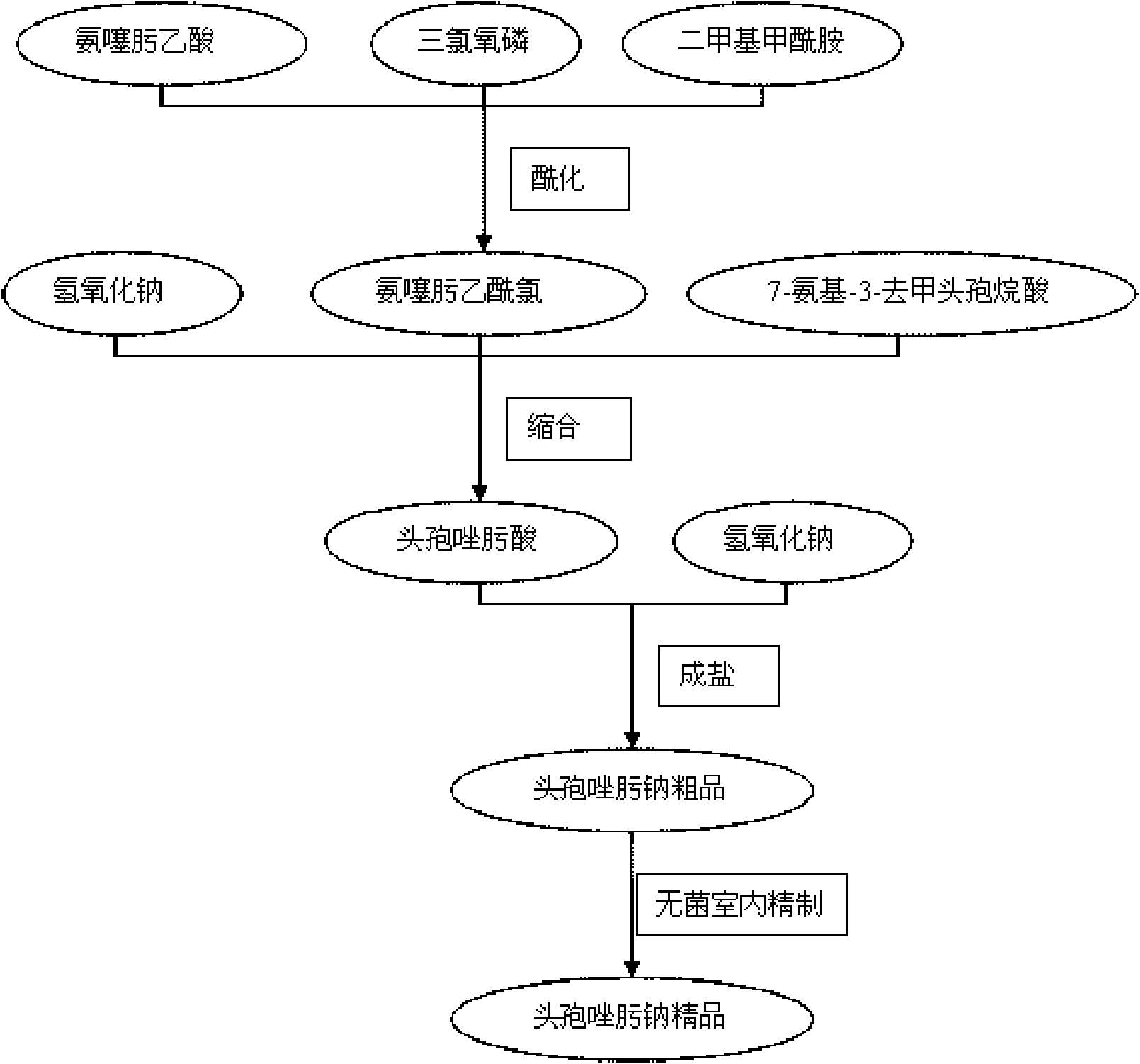
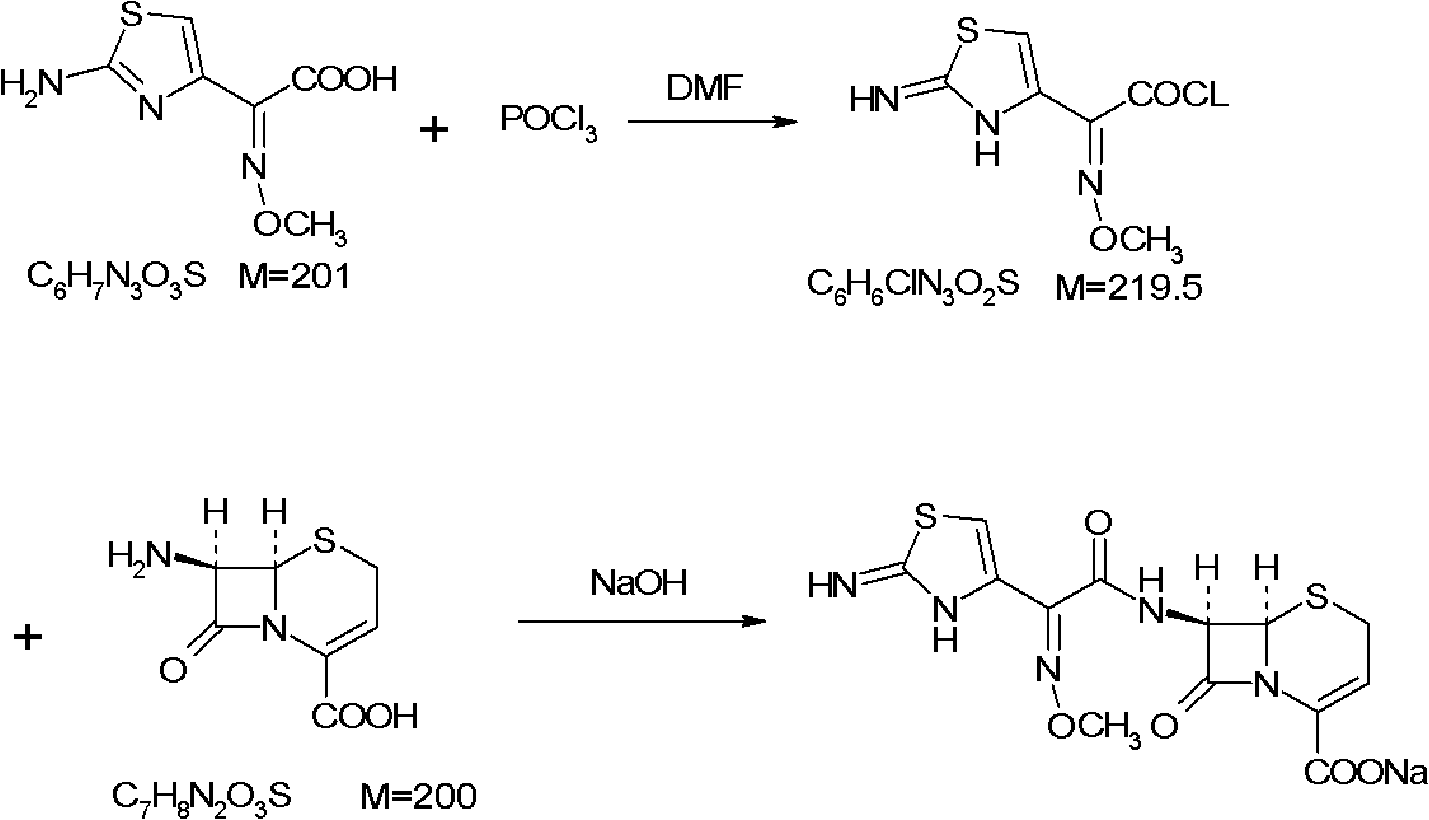
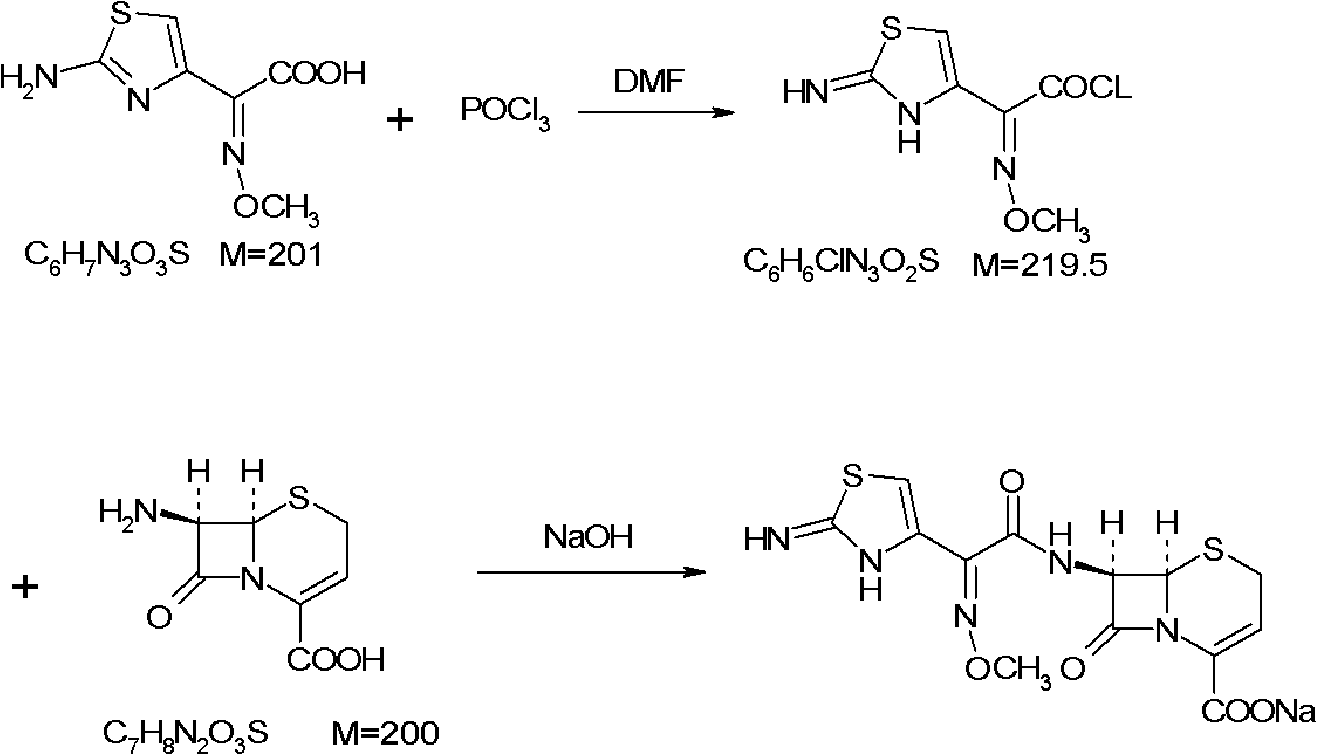
[0114] [embodiment 1] preparation of aminothioxime acetyl chloride
[0115] Put 25.2g of dimethylformamide, 55.1g of phosphorus oxychloride, and 600ml of dry ethyl acetate into the flask. Control the temperature in an ice-water bath at 0-5°C, add 68.1 g of 2-(2-imino-4-thiazolyl)-2-methoxyiminoacetic acid (aminothioxime acetic acid) in batches, and continue the reaction at 0-5°C after adding 1 hour. The solvent was evaporated to dryness under reduced pressure in a water bath at 50°C, and the obtained oil was dissolved in 80ml of acetone for later use.
Embodiment 2
[0116] [Example 2] Preparation of Ceftizoxime Acid
[0117] Add 500ml of water and 14.1g of sodium hydroxide to another flask, and stir to dissolve. Put 67.0 g of 7-amino-3-norcephalosporanic acid into the flask, and adjust the pH value to 12-13 with 5% sodium hydroxide. Cool down to -20~-10°C in an ice-salt bath, and slowly add the prepared aminothioxime acetyl chloride solution dropwise into the flask. During the reaction, control the pH value to 12~13, and the dropwise addition is completed in about 1 hour. After the addition, continue to react at -20~-10°C for half an hour, then raise the temperature to 0°C and react for another hour.
[0118] After the reaction, diethyl ether was added to extract twice, each time with 250 ml of diethyl ether, the layers were separated, and the organic layer was discarded. The aqueous layer was adjusted to pH 1.5 with 10% hydrochloric acid, extracted twice with ethyl acetate, each time with 750 ml of ethyl acetate, and the organic layers...
Embodiment 3
[0119] [Example 3] Preparation of Ceftizoxime Sodium Crude Product
[0120] Put 71.0 g of ceftizoxime acid and 130 ml of water into the flask, control the temperature in an ice-water bath at 0-5°C, and adjust the pH value to 7.0-7.5 with 3 mol / L sodium hydroxide. Add 10.0 g of activated carbon, and stir for 30 minutes at 20-30°C. Suction filtration, and slowly add 1500ml of absolute ethanol to the filtrate under stirring, and the addition is completed in about 30 minutes, and a large amount of white precipitates precipitate out. Refrigerated and crystallized at 0-5°C for 2 hours, filtered, rinsed with 100ml of absolute ethanol, drained, and the filter cake was dried under reduced pressure at 30°C to obtain 62.1g of crude ceftizoxime sodium, with a yield of 82.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com