Ceftizoxime sodium novel crystal form capable of reducing anaphylactic reactions and preparation thereof
A technology of ceftizoxime sodium and crystal, which is applied in the field of injection powder for injection comprising a new crystal form of ceftizoxime sodium and its preparation, and can solve the problems that affect the application of ceftizoxime or its salt, are not suitable for industrialized production, and are complicated to operate. problems, to achieve the effect of improving product stability and drug safety, improving bioavailability, and low residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
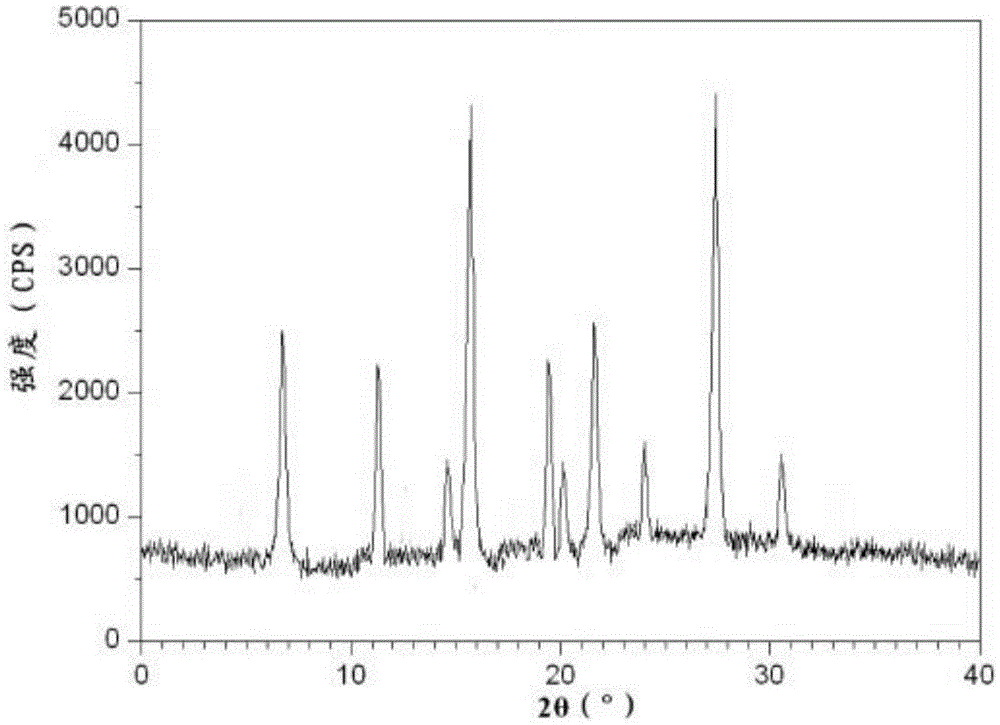
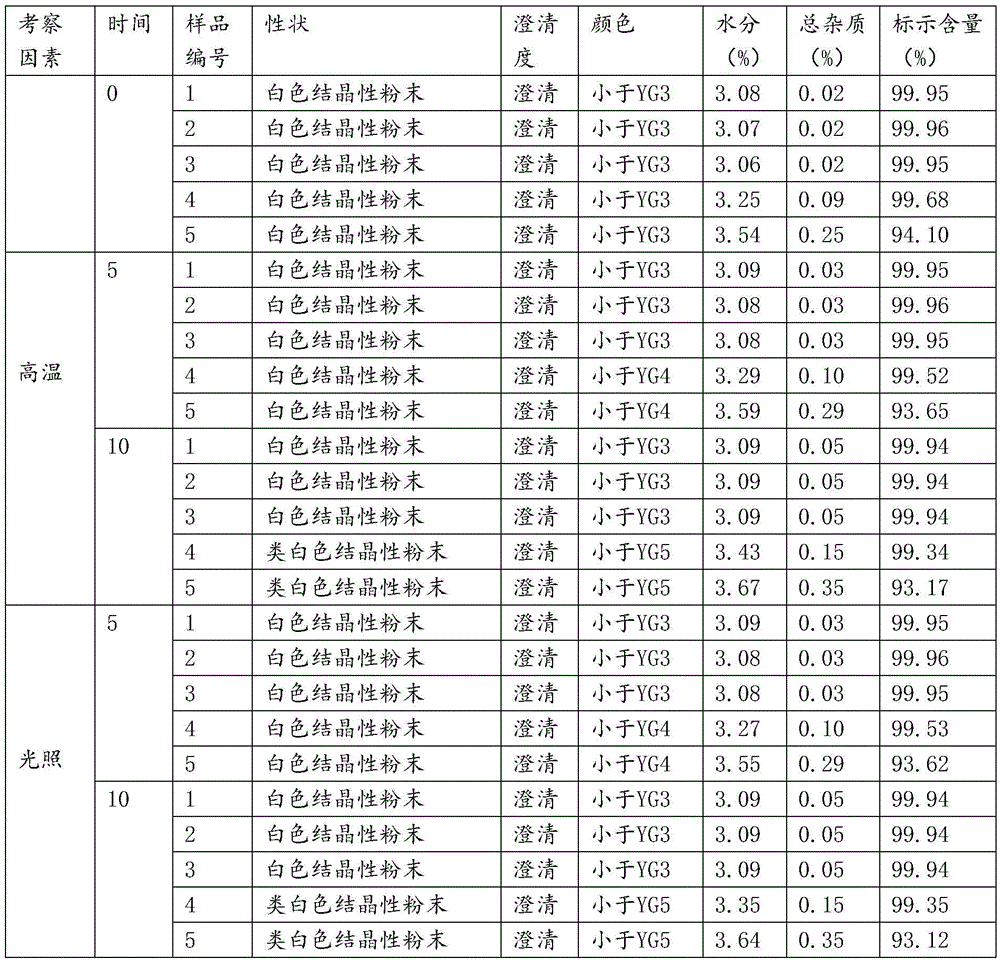
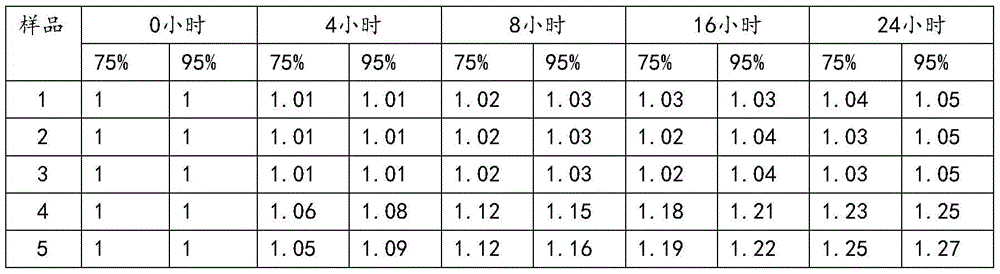
[0022] Dissolve 100 g of the crude product of ceftizoxime sodium in water, control the pH value of the solution to 6.5, and the temperature at 25 ° C, and add dropwise a mixed solvent of isopropanol and tetrahydrofuran (volume ratio 2:1) to the solution under stirring until the solution is cloudy, and the stirring speed is 250 rev / min, the rate of addition is 300ml / h; the temperature is raised to 50°C, and the crystal is left to grow for 3 hours; absolute ethanol is added dropwise under stirring, the stirring speed is 100 rev / min, and the temperature is slowly cooled to 25°C; the rate of addition is 80ml / h, after the dropwise addition, let stand for 2 hours; continue to lower the temperature to 10°C under stirring, let stand for 1 hour; filter, wash with absolute ethanol 3 times, and vacuum-dry at 30°C to obtain ceftizoxime sodium crystals. The particle size is 50-70 μm, the yield is 96.4%, and the HPLC content is 99.95%. The X-ray powder diffraction spectrum obtained by Cu-Kα...
Embodiment 2
[0024] Dissolve 100 g of ceftizoxime sodium crude product in water, control the pH value of the solution to 6.8 and the temperature at 30°C, and add a mixed solvent of isopropanol and tetrahydrofuran (volume ratio 1:1) to the solution dropwise under stirring until the solution is cloudy, and the stirring speed is 250 rpm, the rate of addition is 350ml / h; the temperature is raised to 50°C, and the crystal is left to grow for 3 hours; absolute ethanol is added dropwise under stirring, the stirring speed is 100 rpm, and the temperature is slowly cooled to 25°C; the rate of addition is 80ml / h, after the dropwise addition, let stand for 2 hours; continue to lower the temperature to 10°C under stirring, let stand for 1 hour; filter, wash with absolute ethanol 3 times, and vacuum-dry at 30°C to obtain ceftizoxime sodium crystals. The particle size is 50-70 μm, the yield is 95.9%, and the HPLC content is 99.96%. The X-ray powder diffraction pattern measured by Cu-Kα rays is consistent...
Embodiment 3
[0026] Dissolve 100 g of the crude product of ceftizoxime sodium in water, control the pH value of the solution to 6.3, and the temperature at 25 ° C, and add a mixed solvent of isopropanol and tetrahydrofuran (volume ratio 3:1) to the solution dropwise under stirring until the solution is cloudy, and the stirring speed is 250 rev / min, the rate of addition is 400ml / h; the temperature is raised to 50°C, and the crystal is left to grow for 4 hours; absolute ethanol is added dropwise under stirring, the stirring speed is 100 rev / min, and the temperature is slowly cooled to 25°C; the rate of addition is 80ml / h, after the dropwise addition, let stand for 2 hours; continue to cool down to 10°C under stirring, let stand for 1 hour; filter, wash with absolute ethanol 3 times, and vacuum-dry at 30°C to obtain ceftizoxime sodium crystals. The particle size is 50-70 μm, the yield is 94.6%, and the HPLC content is 99.95%. The X-ray powder diffraction pattern measured by Cu-Kα rays is cons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com