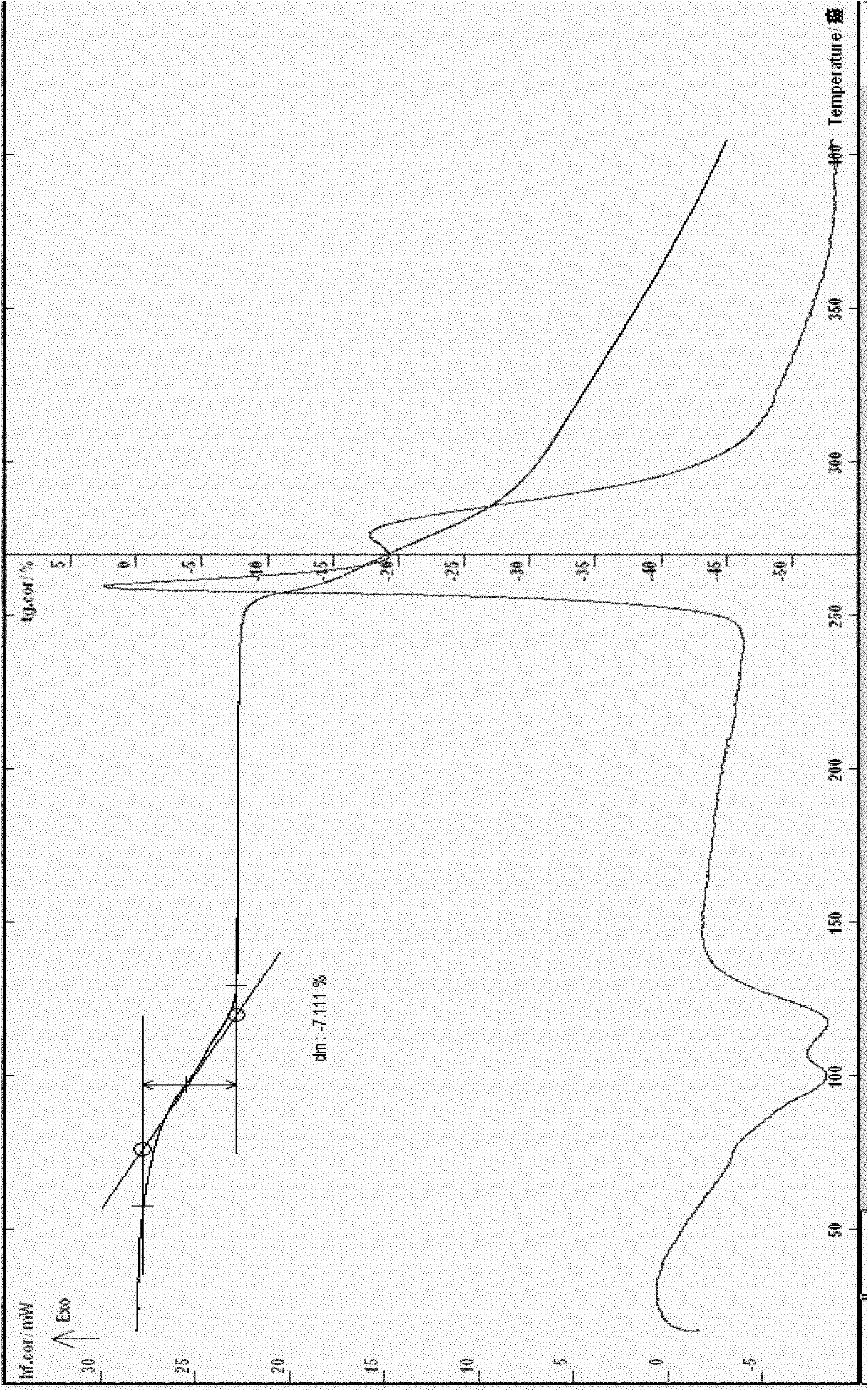
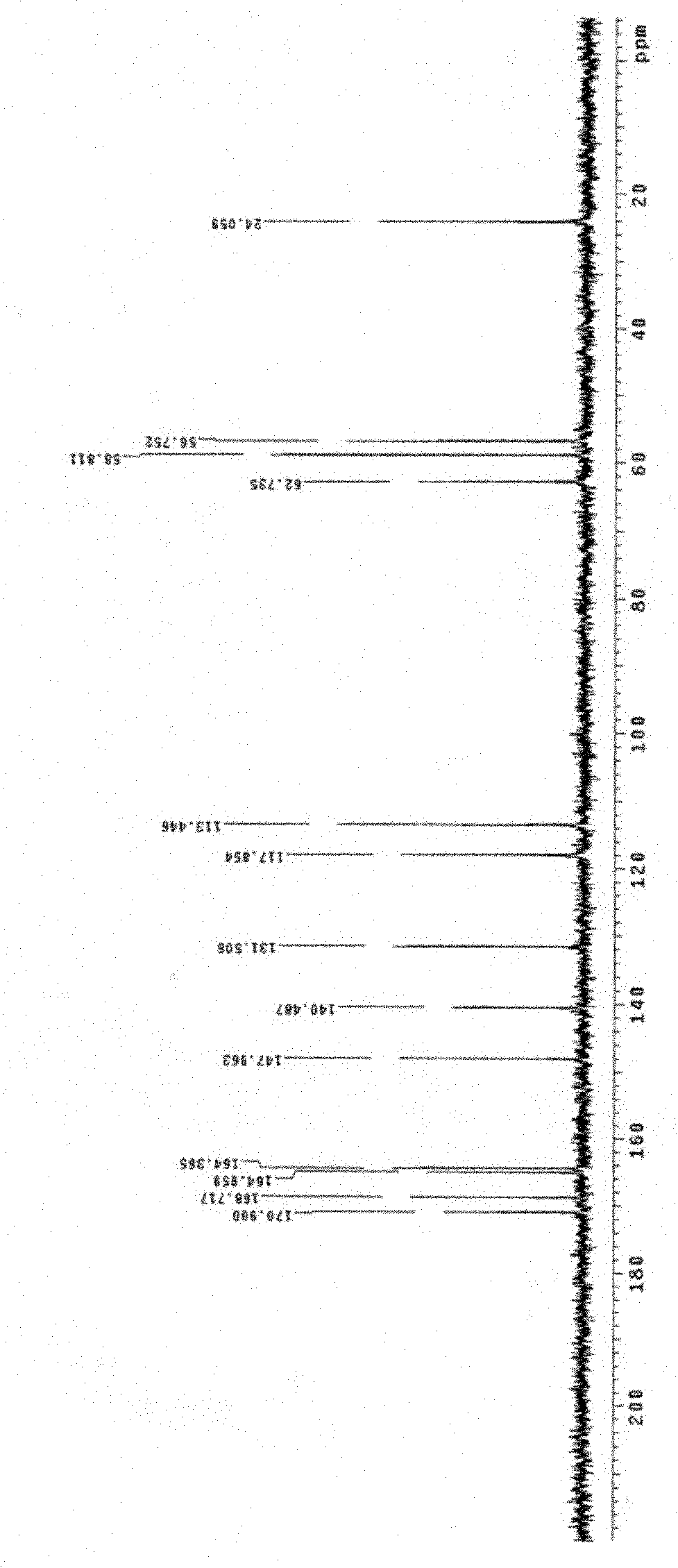
Ceftizoxime sodium crystalline hydrate and preparation method and application thereof
A technology for ceftizoxime sodium and crystalline hydrate, which is applied in the field of ceftizoxime sodium crystalline hydrate and its preparation, can solve the problems such as the preparation method and application of ceftizoxime sodium crystalline hydrate that have not been reported in the literature, and achieves improved Workability, easy storage and transportation, good sliding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Preparation of Ceftizoxime Sodium 1.75 Hydrate
[0057] Add 10g of ceftizoxime acid and 20ml of water to a 50ml Erlenmeyer flask, stir to form a suspension, add 4ml of triethylamine dropwise at 5℃, stir to dissolve, add 0.1g of activated carbon, stir for 30 minutes, filter with suction, wash with water, and pump Filter, add dropwise 20ml sodium isooctanoate and ethanol mixed solution (containing 6.5g sodium isooctanoate) to the filtrate at 5℃, stir, adjust the pH to about 7.0 with glacial acetic acid, slowly add 50ml acetone and 250ml ethanol, and place below 5℃ , The solid is fully analyzed, filtered with suction, washed 3 times with a small amount of ethanol, and filtered with suction. The obtained solid is recrystallized with about 20ml of water, 260ml of ethanol, and 20ml of acetone as solvents. Wash, filter with suction, and vacuum dry the obtained solid at about 40°C for about 4 hours to obtain 5.8g off-white solid, melting point: 205°C decomposition (ELECT...
Embodiment 2
[0058] Example 2 Preparation of Ceftizoxime Sodium 1.75 Hydrate
[0059] Add 20g of ceftizoxime acid and 50ml of water to a 250ml Erlenmeyer flask, stir to form a suspension, add dropwise 20ml of anhydrous sodium carbonate 2.9g aqueous solution at 15°C, stir to dissolve, add 0.2g activated carbon, and stir for 30 minutes. Suction filtration, water washing, suction filtration, the filtrate is adjusted to pH 6.9 with glacial acetic acid, and then slowly dripped with 100ml of acetone and 250ml of ethanol, placed below 5℃, the solid is fully analyzed, filtered with suction, washed with a small amount of ethanol 3 times, filtered with suction, The obtained solid was recrystallized with 30ml of water, 300ml of ethanol, 20ml of isopropanol, and 5ml of isopropyl ether as the crystallization solvent. Place it below 5°C to allow the crystals to be fully analyzed, filtered with suction, washed with a small amount of dichloromethane, filtered with suction, and vacuum at around 40°C. After dr...
Embodiment 3
[0060] Example 3 Preparation of Ceftizoxime Sodium 1.5 Hydrate
[0061] Add 10g of ceftizoxime and 30ml of water to a 250ml Erlenmeyer flask, stir to form a suspension, add dropwise saturated aqueous sodium bicarbonate solution at 10°C until the pH is about 7.0, stir to dissolve, add 0.2g of activated carbon, and stir for 30 minutes , Suction filtration, washing with water, suction filtration, slowly dropwise add 20ml of chloroform and 300ml of ethanol to the filtrate, and place below 10℃ to allow the solids to be fully analyzed. Filter with suction, wash with a small amount of ethanol 3 times, and filter with suction. The obtained solids are 18ml of water and 300ml of ethanol. , 20ml of chloroform is the crystallization solvent for recrystallization, placed below 8℃, the crystals are fully analyzed, filtered with suction, washed with a small amount of dichloromethane, and dried under vacuum at 42℃ for about 6 hours to obtain 5.6g off-white solid, melting point: about 194℃ slightl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com