Ceftizoxime sosium compound of new way
A kind of technology of ceftizoxime sodium and compound, applied in the field of drug synthesis, can solve the problems such as no literature and patent reports etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
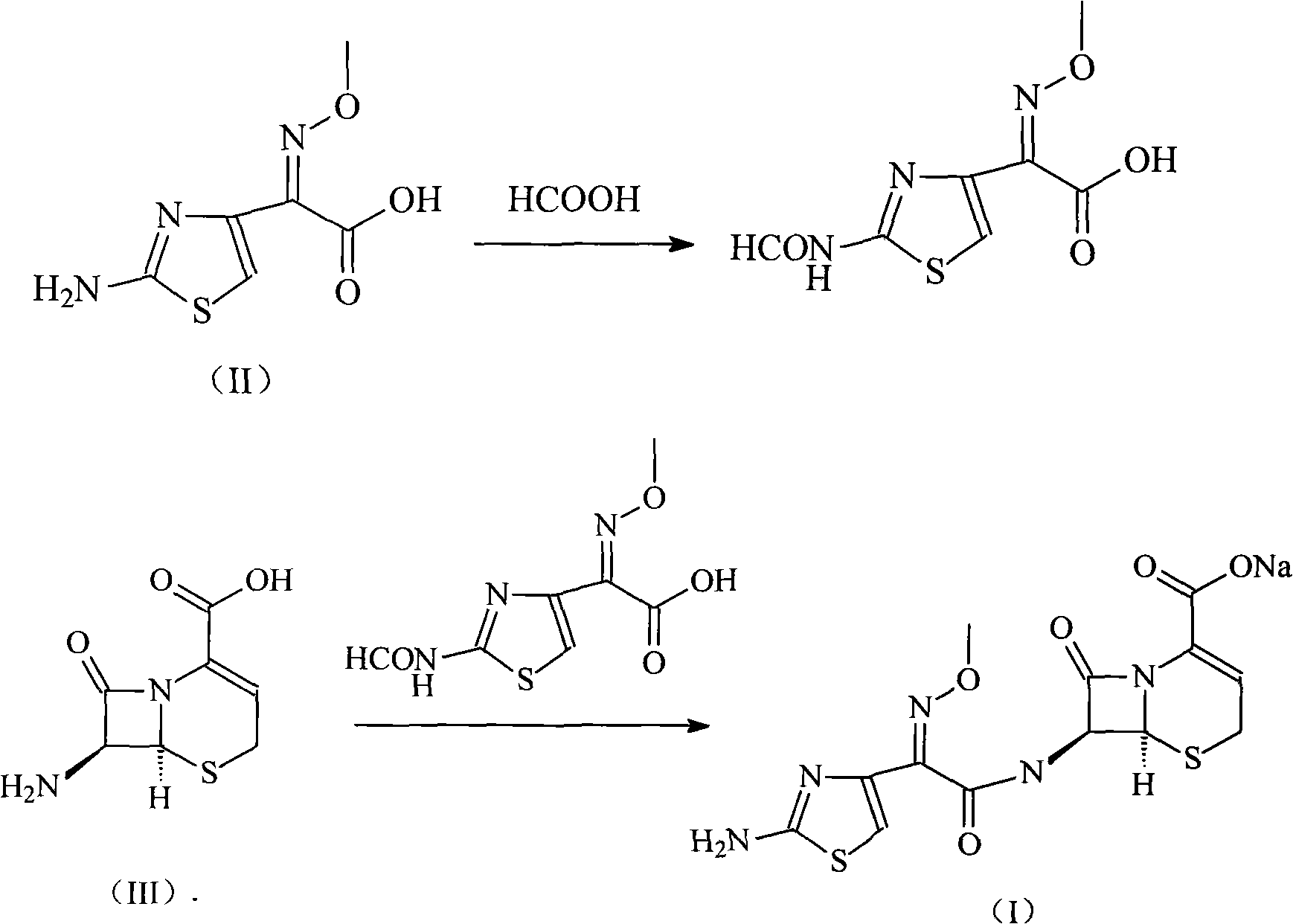
[0026] Example 1 Synthesis of 2-(2-formylaminothiazol-4-yl)-2-methoxyiminoacetic acid
[0027] Add 201 grams of aminothioxamic acid to 2 liters of formic acid, add 30 grams of 4A molecular sieves as a catalyst, heat up to 50 ° C for 4 hours, distill most of the formic acid under reduced pressure, add 1 liter of water, stir, and extract with 800 ml of ethyl acetate. Dry over sodium sulfate and concentrate under reduced pressure to obtain 217 g of the product 2-(2-formylaminothiazol-4-yl)-2-methoxyiminoacetic acid with a yield of 94.6%.
Embodiment 2
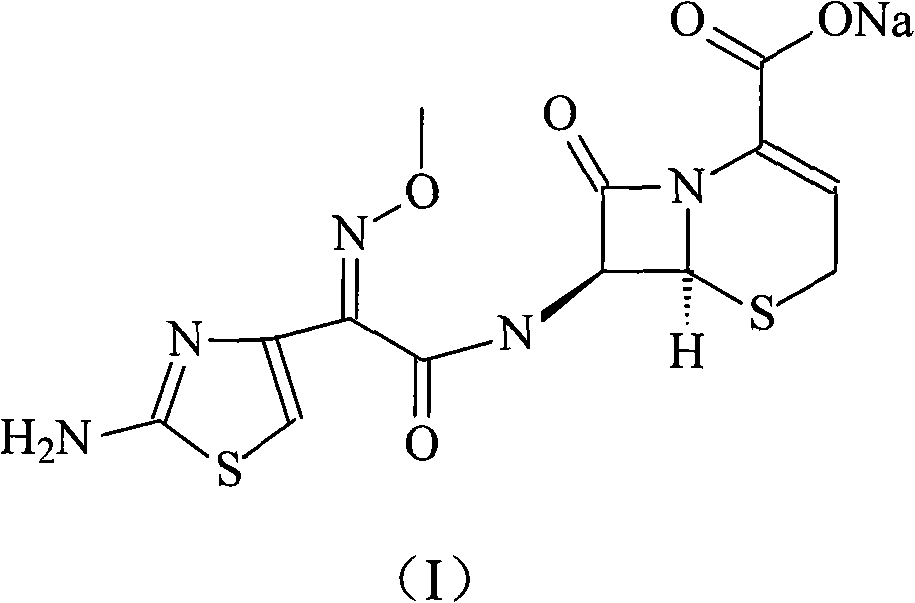
[0028] The synthesis of embodiment 2 ceftizoxime sodium
[0029] Dissolve 485 grams of triphenylphosphine in 1 liter of 1,2-ethylene dichloride, add dropwise to 1 liter of 2-ethylene dichloride containing 524 grams of triphosgene, and keep the reaction temperature at 5°C, after the dropwise addition, react at this temperature for 2 hours, then add the mixed solution dropwise to 200 grams of 2-(2-formylaminothiazol-4-yl)-2-methoxyimine acetic acid 800ml of 1,2-ethylene dichloride, reacted at 5°C for 1.5 hours, then this mixed solution was added dropwise to 2 In 1 liter of aqueous solution, the temperature was controlled at 0°C, and the pH of the reaction solution was controlled by sodium hydroxide solution to be 11. After the dropwise addition, the reaction was carried out for 1 hour, and then the pH was adjusted to 3 with hydrochloric acid, separated into layers, dried with anhydrous sodium sulfate, and reduced Concentrate under pressure, dissolve the concentrated product in ...
Embodiment 3
[0037] Example 3 Synthesis of 2-(2-formylaminothiazol-4-yl)-2-methoxyiminoacetic acid
[0038] Add 402 grams of aminothioxamic acid to 4 liters of formic acid, add 60 grams of 4A molecular sieves as a catalyst at the same time, heat up to 50 ° C for 4 hours, distill most of the formic acid under reduced pressure, add 2 liters of water, stir, and extract with 1500 ml of ethyl acetate. Dry over sodium sulfate and concentrate under reduced pressure to obtain 430.3 g of the product 2-(2-formylaminothiazol-4-yl)-2-methoxyiminoacetic acid with a yield of 93.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com