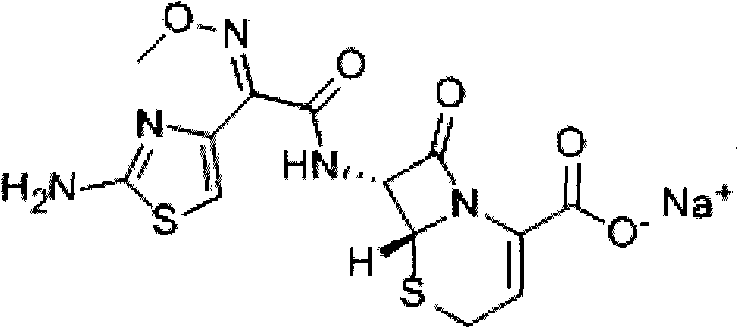
Method for preparing ceftizoxime powder injection for injection and use thereof
A technology for ceftizoxime sodium and injection, which is applied in the field of preparation of ceftizoxime sodium powder injection preparations for injection, can solve the problems of reduced stability, high oxygen content in the bottle, influence and the like, and achieves increased stability of the finished product, The effect of improving product stability and reducing oxygen content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
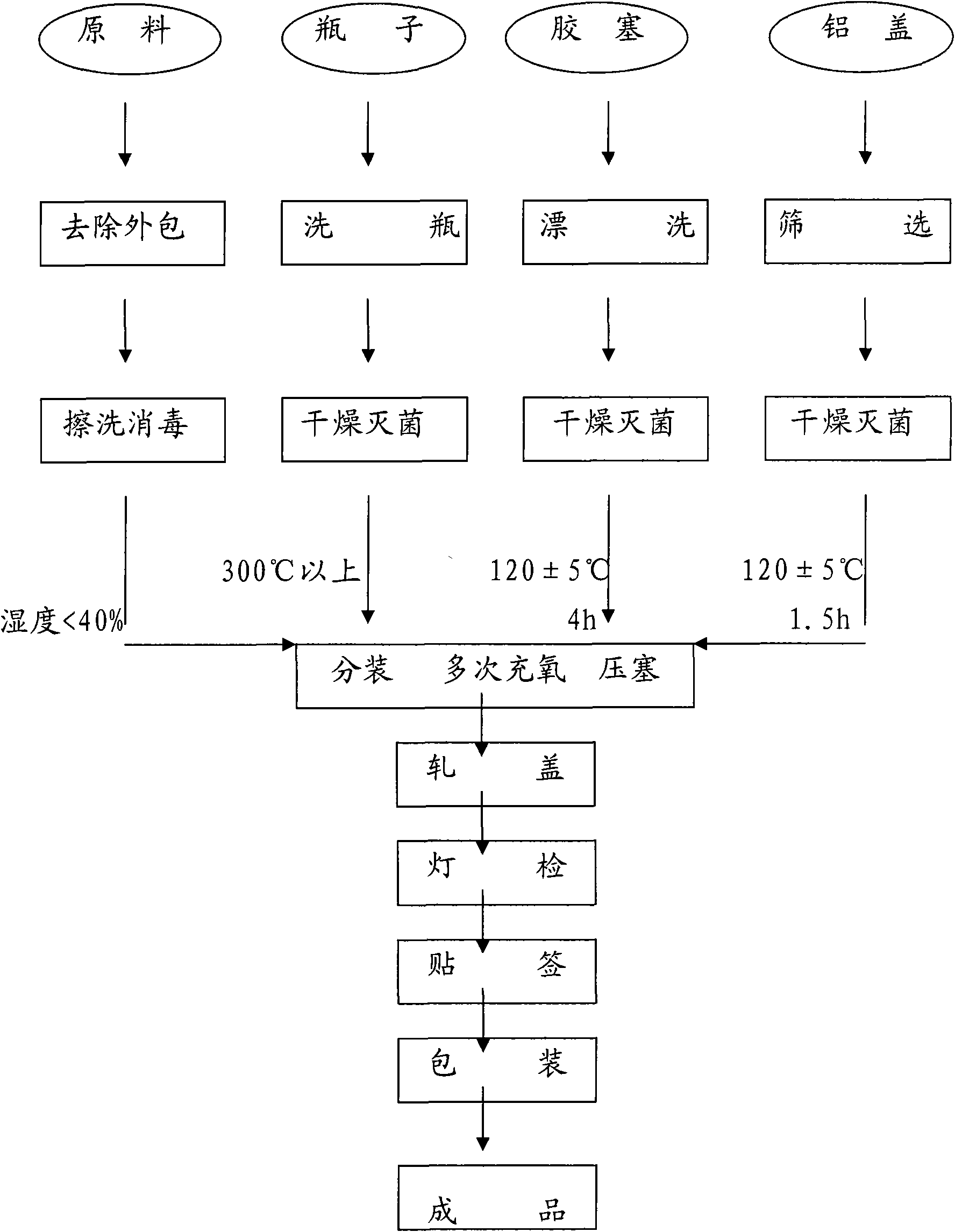
[0038] In this example, the ceftizoxime sodium powder injection preparation method is used in the preparation method of the ceftizoxime sodium powder injection preparation method provided by the present invention to prepare the ceftizoxime sodium powder injection preparation by means of airflow. The specific steps are described in detail as follows.
[0039] 1) Wash the bottles with purified water through a bottle washer and blow them dry, then enter the laminar flow sterilization tunnel and be sterilized and cooled at a high temperature above 300°C. Check the purified water for visible foreign matter before cleaning the bottle and rubber stopper, and use it only after passing the inspection.
[0040]2) Clean the rubber stopper, rinse the rubber stopper with purified water and water for injection, and after passing the inspection according to the quality standard, use RXH-1 hot air circulation oven (Macofar, Italy) for dry heat sterilization at 122±5℃ for 4 hours, standby .
[004...
Embodiment 2
[0048] In this example, the ceftizoxime sodium powder injection preparation is prepared by the method of screw sub-packing in the preparation method of the ceftizoxime sodium powder injection preparation provided by the present invention. Except for the sub-packaging method, the other specific steps are the same as in Example 1.
[0049] Check the purified water for visible foreign matter before cleaning the bottle and rubber stopper, and use it only after passing the inspection. Wash the rubber stopper, after passing the inspection according to the internal control quality standard, dry heat sterilization for 4 hours. The aluminum plastic cover is sterilized by dry heat. Wash the bottle. The cleaned bottles shall be sterilized after passing the inspection according to the internal control quality standard.
[0050] Raw materials are pre-packed; bottles and rubber stoppers are before and after sterilization; related items (such as visible foreign matter, clarity, color of raw mate...
Embodiment 3
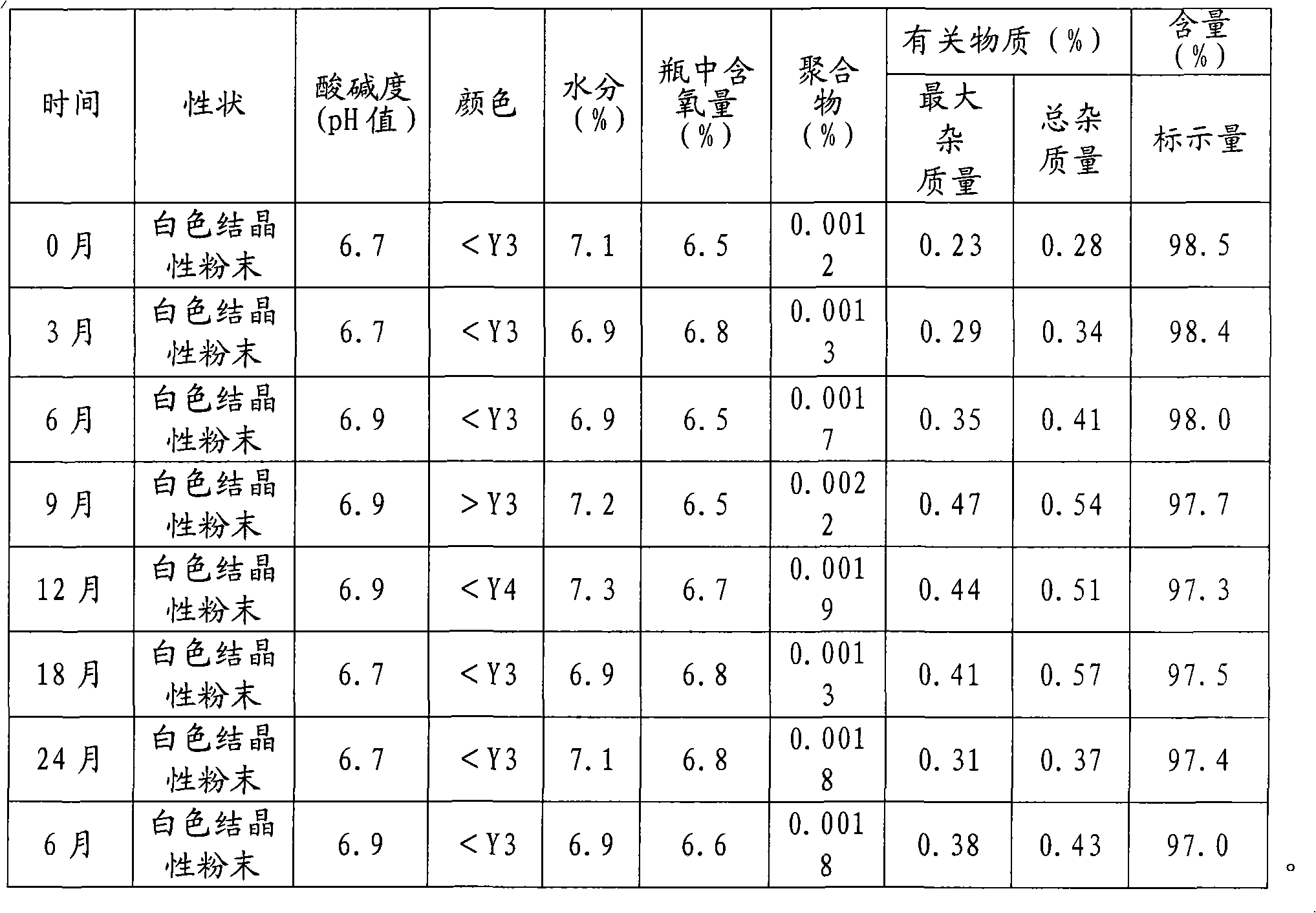
[0054] In this example, the stability of the ceftizoxime sodium powder injection preparation prepared by the preparation method of the ceftizoxime sodium powder injection preparation provided by the present invention was carried out. The specific determination method refers to the national drug standard YBH02832004.
[0055] Take trial samples (30 bottles, 1.0g / bottle), and place them under the conditions of temperature 25℃±2℃ and relative humidity 60%±10% for long-term stability test. At the end of the 3rd, 6th, 12th, 24th, and 36th months, each survey item was tested according to the survey method, and compared with the results in 0 months. The content determination was calculated according to the external standard method, and the calculation of related substances was based on self-control. According to the calculation method, the polymer is the impurity specified in the standard. The results are shown in Table 1.
[0056] Table 1 Long-term test results of ceftizoxime sodium for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com