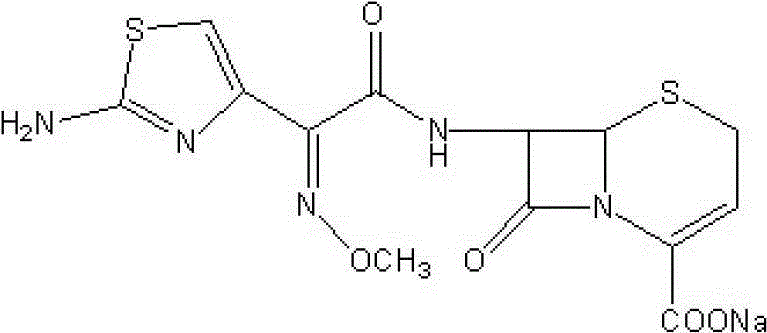
Ceftizoxime sodium for injection and preparation method thereof as well as synthetic method for ceftizoxime sodium serving as crude drug
A technology of ceftizoxime and ceftizoxime sodium, which is applied in the field of medicine, can solve problems such as accelerated dissolution rate, reduced packaging efficiency, and slowed dissolution rate, so as to overcome poor clarity, improve stability, and improve solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
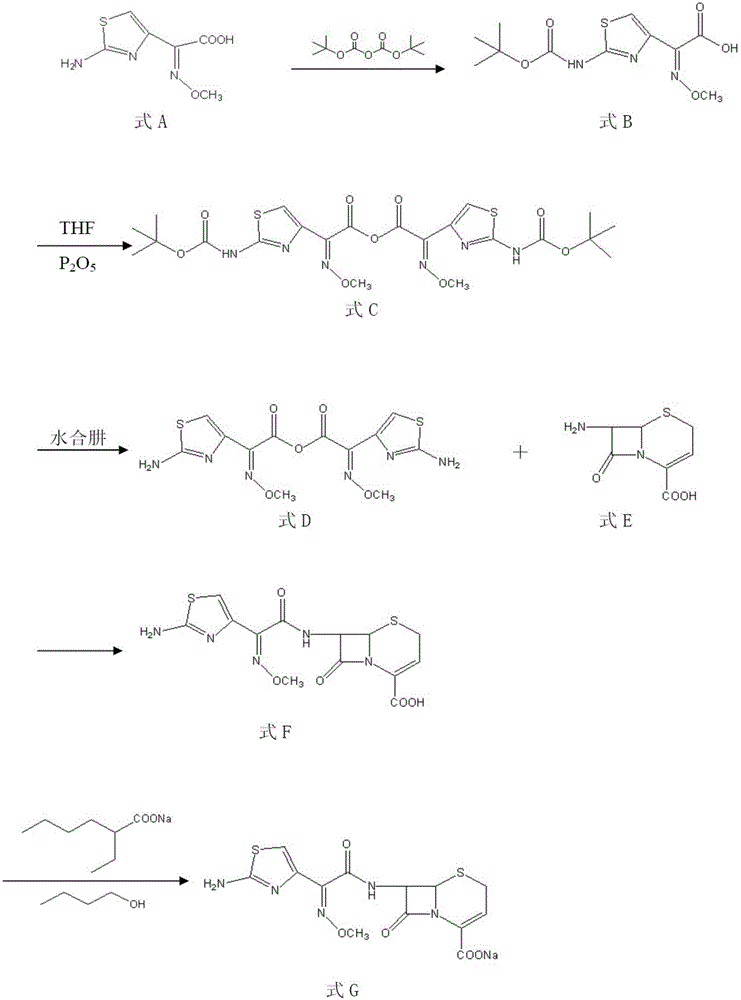
[0060] The synthetic method of crude drug ceftizoxime sodium is characterized in that the method comprises the following steps:
[0061] 1) Preparation of compound tert-butoxycarbonylaminothioxime acetic acid as shown in formula B
[0062] Add 100g of the compound 2-(2-amino-4-thioxime)-2-methoxyiminoacetic acid (aminothioxime acetic acid) shown in formula A into the dioxane-water mixed solution (dioxane : Dissolve in 500ml of water (2:1), adjust the pH to 9 with triethylamine, add 108g of di-tert-butyl dicarbonate to the ice-water bath, react for 6h, adjust the pH to 4.5 with hydrochloric acid, and precipitate a white solid, filter with suction, 50ml of chloroform Rinse the filter cake 2 times, and dry under reduced pressure to obtain 138 g of compound tert-butoxycarbonylaminothioxime acetic acid shown in formula B, yield 92.2%;
[0063] Formula A:
[0064] Formula B:
[0065] 2) Preparation of compound tert-butoxycarbonylaminothioxime acetic anhydride as shown in form...
Embodiment 2
[0088] The synthetic method of crude drug ceftizoxime sodium is characterized in that the method comprises the following steps:
[0089] 1) Preparation of compound tert-butoxycarbonylaminothioxime acetic acid as shown in formula B
[0090] Add 100g of the compound 2-(2-amino-4-thioxime)-2-methoxyiminoacetic acid (aminothioxime acetic acid) shown in formula A into the dioxane-water mixed solution (dioxane : Dissolve in 500ml of water (2:1), adjust the pH to 10 with triethylamine, add 108g of di-tert-butyl dicarbonate to the ice-water bath, react for 8h, adjust the pH to 5.5 with hydrochloric acid, and precipitate a white solid, filter with suction, 50ml of chloroform Rinse the filter cake 2 times, and dry under reduced pressure to obtain 148 g of compound tert-butoxycarbonylaminothioxime acetic acid as shown in formula B, with a yield of 98.9%;
[0091] Formula A:
[0092] Formula B:
[0093] 2) Preparation of compound tert-butoxycarbonylaminothioxime acetic anhydride as...
Embodiment 3
[0116] The synthetic method of crude drug ceftizoxime sodium is characterized in that the method comprises the following steps:
[0117] 1) Preparation of compound tert-butoxycarbonylaminothioxime acetic acid as shown in formula B
[0118] Add 100g of the compound 2-(2-amino-4-thioxime)-2-methoxyiminoacetic acid (aminothioxime acetic acid) shown in formula A into the dioxane-water mixed solution (dioxane : Dissolve in 500ml of water (2:1), adjust the pH to 10 with triethylamine, add 108g of di-tert-butyl dicarbonate to the ice-water bath, react for 8h, adjust the pH to 5.5 with hydrochloric acid, precipitate a white solid, filter it with suction, and add 50ml of chloroform Rinse the filter cake 2 times, and dry under reduced pressure to obtain 148 g of compound tert-butoxycarbonylaminothioxime acetic acid as shown in formula B, with a yield of 98.9%;
[0119] Formula A:
[0120] Formula B:
[0121] 2) Preparation of compound tert-butoxycarbonylaminothioxime acetic anhyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com