Preparation method of ceftizoxime sodium
A technology of ceftizoxime sodium and ceftizoxime acid, which is applied in the field of preparation of ceftizoxime sodium, can solve the problems of increasing operating procedures and production costs, many side reactions, prolonging reaction steps, etc., and achieves a simple and easy preparation process, Low cost, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
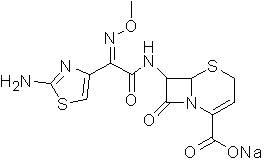
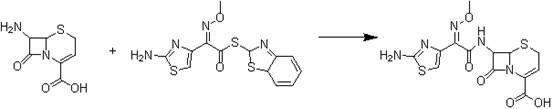
[0018] A preparation method of ceftizoxime sodium, comprising (1) 7-ANCA as a raw material, and 2-(2-amino-4-thiazolyl)-2-methoxyimine-acetyl-benzothiazole thioester (AE -Active ester) is condensed in a solvent and in the presence of an alkali to obtain the ceftizoxime acid intermediate; (2) the ceftizoxime acid intermediate is reacted with the salt-forming agent sodium hydroxide to obtain ceftizoxime sodium,
[0019] In step (1), the specific process is to add 7-ANCA to the solvent, cool down to 0-5°C, add AE-active ester, raise the temperature to 20-40°C, and react for 4-5h. After the reaction, the reaction liquid Pour into ethyl acetate to stir, let stand to separate layers, extract the organic layer with water twice, combine the water layer, add the adsorbent, continue to stir for 2-3h, filter, and the filtrate is at 0-5°C. Adjust the pH to 5-6 with 10-15% hydrochloric acid, and precipitate a white solid. Stir the cultured crystals in an ice bath at 0-5°C for 2-3 hours, fi...
Embodiment 1
[0026] Synthesis of ceftizoxamic acid:
[0027] Take a 200ml three-necked flask, add 4g of 7-ANCA, add a mixture of 25ml of water and 40ml of tetrahydrofuran, stir and cool down to 0°C, add 4.7g of triethylamine dropwise and the solution becomes clear, and then add 14.6g of AE active ester , heated up to 20°C, stirred for 4 hours, poured the reaction solution into 100ml of ethyl acetate and stirred, statically separated the liquid, extracted the organic layer twice with 100ml of water, combined the aqueous layer, added the adsorbent, and continued to stir for 2- 3h, filter, the filtrate is at 0-5°C, use hydrochloric acid with a mass percent solubility of 10-15% to adjust the pH to 5-6, and a white solid is precipitated, stir the crystals in an ice bath at 0-5°C for 2-3h, filter , 50 milliliters of water and 50 milliliters of acetone washed the filter cake successively to obtain 6.58 g of ceftizoxamic acid intermediate, with a yield of 86% and a purity of 99.12% by HPLC norm...
Embodiment 2
[0029] Ceftizoxime Sodium
[0030] Add 1 clozoxamic acid and 20ml ethanol to a 200ml single-mouth bottle, add 1N sodium hydroxide dropwise under ice bath, adjust the pH to 8, at this time the solution is clear, then slowly add 160ml of acetone dropwise, white crystals are precipitated, stir for 30 minutes, filtered with suction, washed with acetone, and dried to obtain 0.84 g of a white solid with a yield of 80% and a purity of 99.05% by HPLC normalization method.
[0031] 1H-NMR (DMSO-d6) δ: 3.35(2H, m), 3.47(3H, s), 4.95(1H, d, J=3.9 Hz), 5.62(1H, dd, J = 3. 8 Hz, 6. 6 Hz), 5. 96 (1H, m), 6. 71 (1H, s), 7. 23 (2H, s), 9. 51 (1H, d, J =6. 6 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com