Ceftizoxime sodium liposome injection
A technology of cefizoxime sodium and liposome, applied in the field of medicine, can solve the problems of low bioavailability, poor stability, poor reconstitution and the like, and achieve the effects of high bioavailability, good stability and good resolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
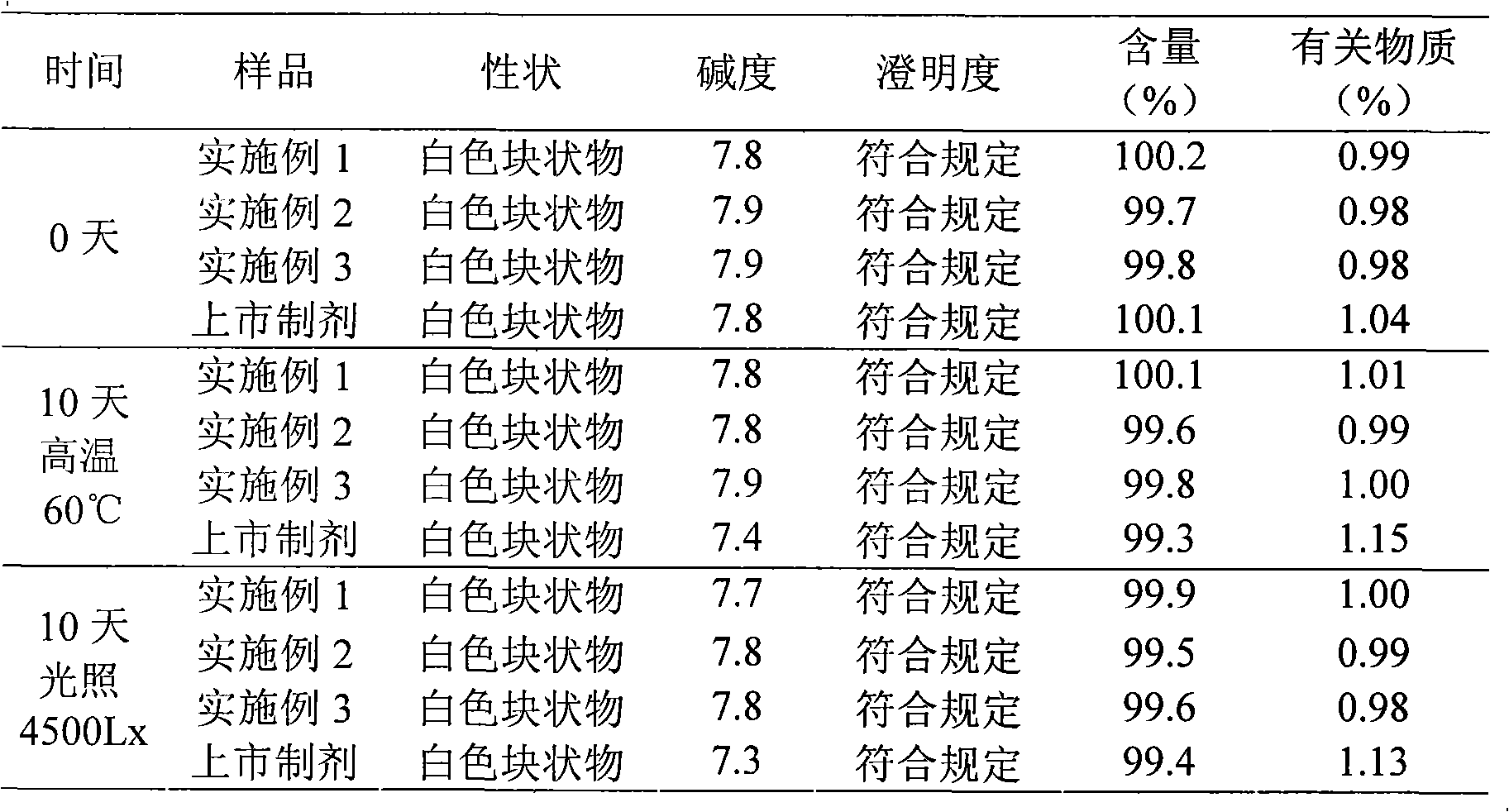
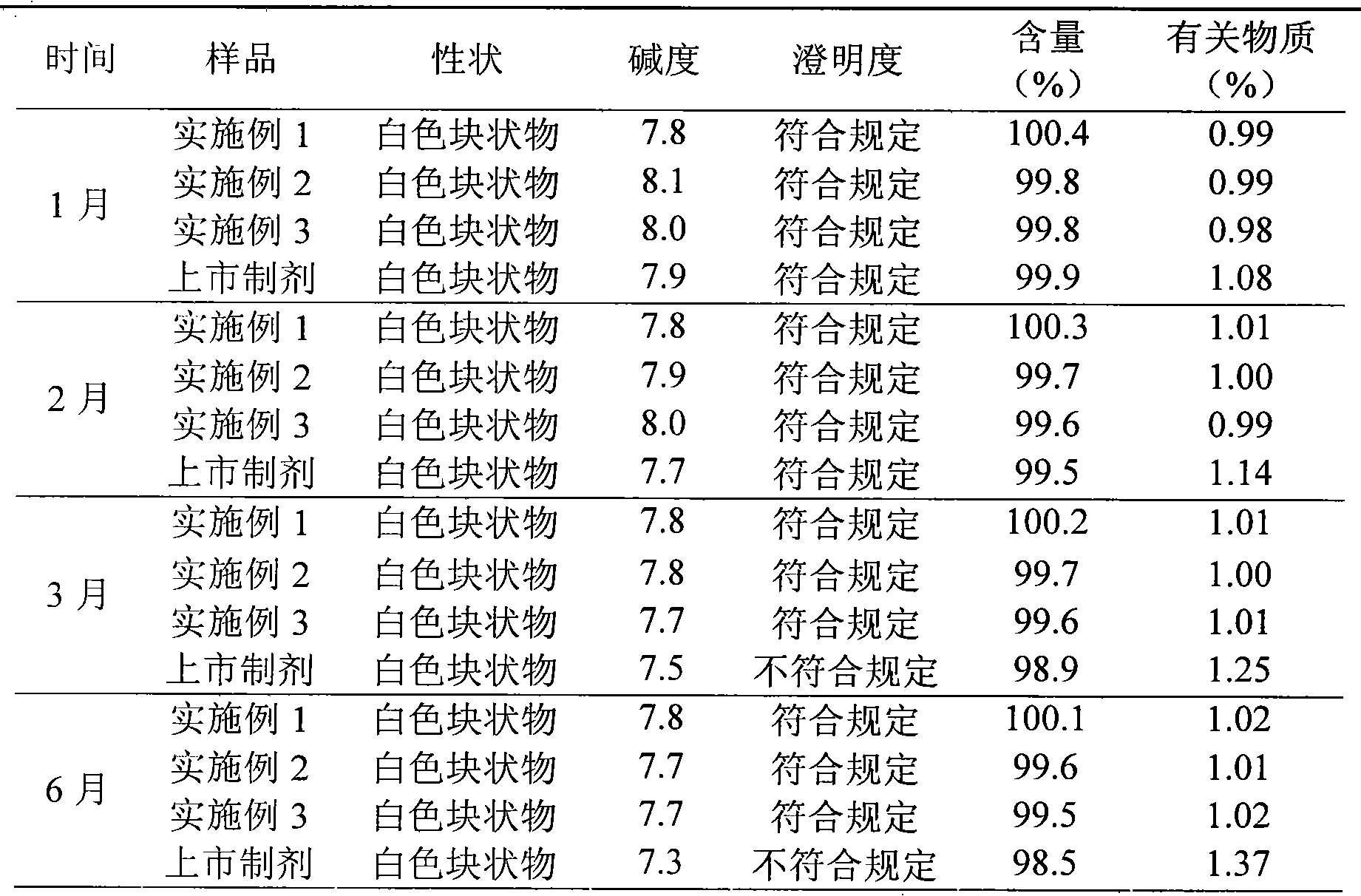
Embodiment 1
[0030] The preparation of embodiment one ceftizoxime sodium liposome lyophilized preparation
[0031] Prescription (specification 0.05g)
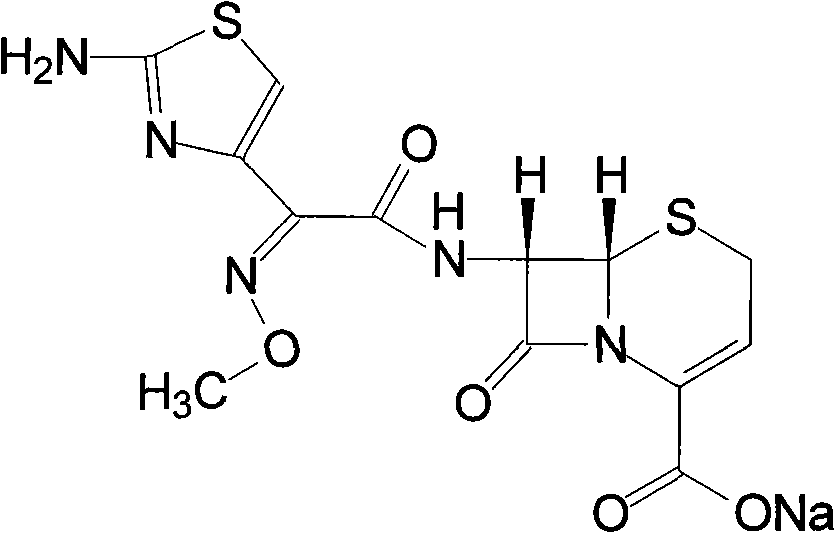
[0032] Ceftizoxime Sodium 5g
[0033] Soy Lecithin 23g
[0034] Cholesterol 23g
[0035] Sodium dihydrogen phosphate 5g
[0036] Disodium hydrogen phosphate 15g
[0037] Sorbitol 18g
[0038] Vitamin E 7g
[0039] Diethyl ether 200ml
[0040] Preparation Process:
[0041] A Dissolve 5g of sodium dihydrogen phosphate and 15g of disodium hydrogen phosphate in 100ml of water for injection to make a phosphate buffer, and adjust the pH to 7.0 with 0.1mol / L sodium hydroxide solution and 1% phosphoric acid solution;
[0042] B Melt 23g of soybean lecithin and 23g of cholesterol in 200ml of ether at 70°C, add phosphate buffer with a pH value of 7.0, stir and mix evenly, wait until the organic solvent is completely evaporated, transfer to a tissue masher and stir at high speed 10-30min, filter and sterilize;
[0043] C, add 5g of ceftizoxi...
Embodiment 2
[0044] The preparation of embodiment biscetizoxime sodium liposome lyophilized preparation
[0045] Prescription (specification 0.1g)
[0046] Ceftizoxime Sodium 10g
[0048] Cholesterol 27g
[0049] Sodium dihydrogen phosphate 15g
[0050] Disodium hydrogen phosphate 45g
[0052] Sodium sulfite 9g
[0053] Isopropanol 400ml
[0054] Preparation Process:
[0055]A Dissolve 15g sodium dihydrogen phosphate and 45g disodium hydrogen phosphate in 300ml water for injection to make phosphate buffer, and adjust the pH to 6.5 with 0.1mol / L sodium hydroxide solution and 1% phosphoric acid solution;
[0056] B Melt 27g of egg yolk lecithin and 27g of cholesterol in 400ml of isopropanol at 70°C, add phosphate buffer with a pH value of 6.5, stir and mix evenly, and transfer to a tissue masher after the organic solvent has evaporated completely Stir at high speed for 10-30 minutes, filter and sterilize;
[0057] C, after...
Embodiment 3
[0058] The preparation of embodiment three ceftizoxime sodium liposome freeze-dried preparation
[0059] Prescription (specification 0.2g)
[0060] Ceftizoxime Sodium 20g
[0061] Soy Lecithin 18g
[0062] Cholesterol 18g
[0063] Sodium dihydrogen phosphate 5g
[0064] Disodium hydrogen phosphate 15g
[0065] Mannitol 16g
[0066] Thiourea 6g
[0067] Diethyl ether 200ml
[0068] Preparation Process:
[0069] A Dissolve 5g of sodium dihydrogen phosphate and 15g of disodium hydrogen phosphate in 100ml of water for injection to make a phosphate buffer, and adjust the pH to 7.8 with 0.1mol / L sodium hydroxide solution and 1% phosphoric acid solution;
[0070] B Melt 18g of soybean lecithin and 18g of cholesterol in 200ml of ether at 70°C, add phosphate buffer with a pH value of 7.8, stir and mix evenly, wait until the organic solvent is completely evaporated, transfer to a tissue masher and stir at high speed 10-30min, filter and sterilize;
[0071] C, after adding 20g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com