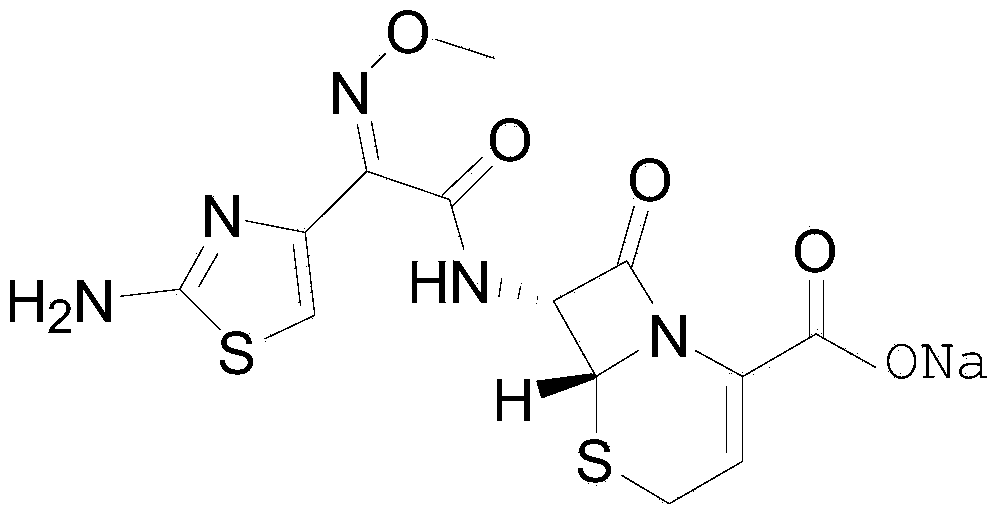
Ceftizoxime sodium compound crystal form, and preparing method and pharmaceutical preparation thereof
A technology for cefizoxime sodium and pharmaceutical preparations, which is applied in the field of drug synthesis, can solve problems such as affecting drug safety and increasing body irritation, so as to improve product stability and drug safety, reduce irritation, and achieve a simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation method of a crystal form of ceftizoxime sodium compound of this embodiment comprises the following steps: take 10 g of ceftizoxime sodium raw material (Harbin Pharmaceutical Group Pharmaceutical General Factory, purity 93%), add 60 ml of pure water, stir at room temperature (100 turn / min) to completely dissolve, and control the pH value to 6.5; then slowly add 300ml of absolute ethanol into the above container within 50min, and make it completely precipitate at 25°C, and then filter and dry to constant weight at 45°C ℃, -0.05MPa, dried for 4 hours; 7.8g of fine granular ceftizoxime sodium compound crystal form was obtained.
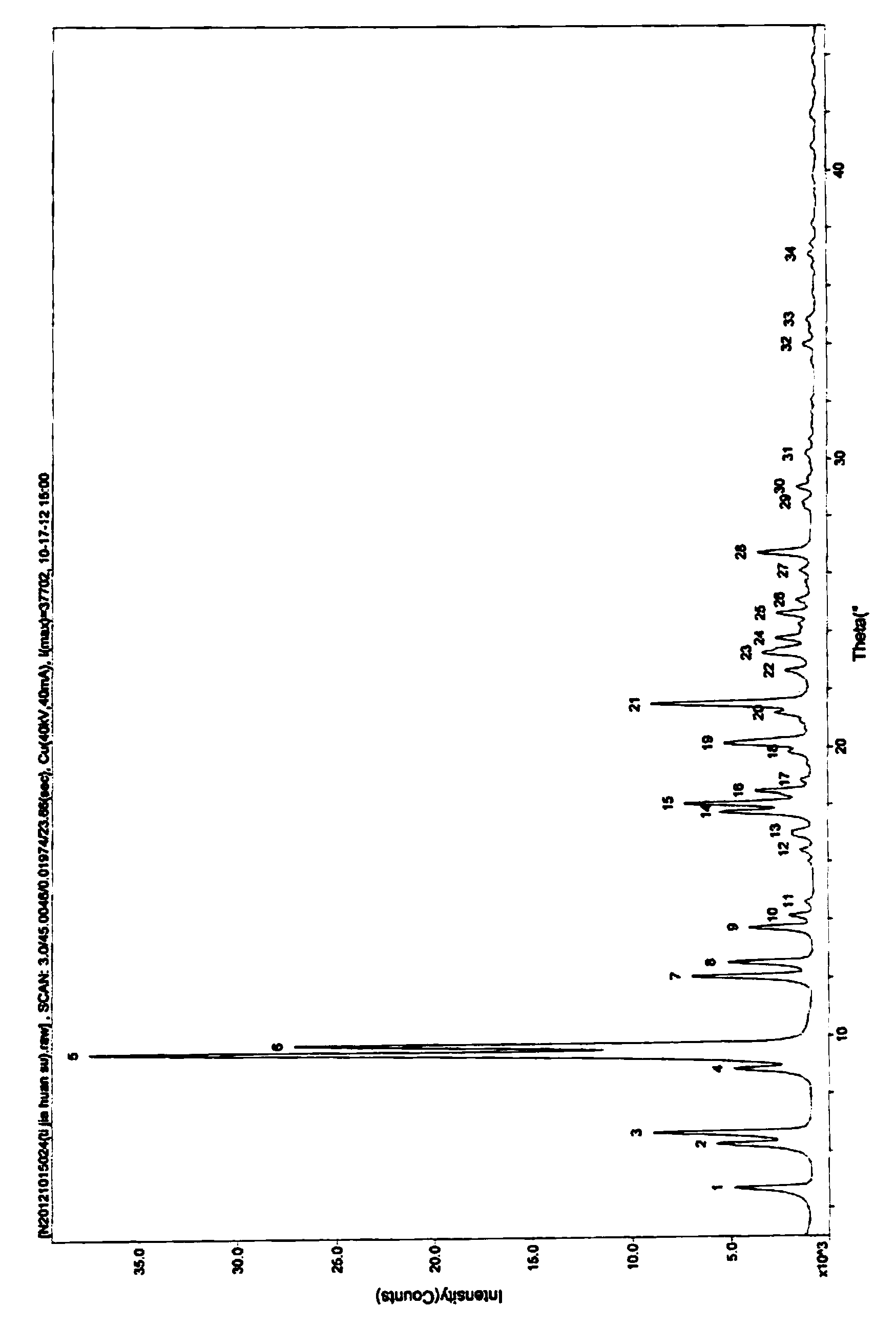
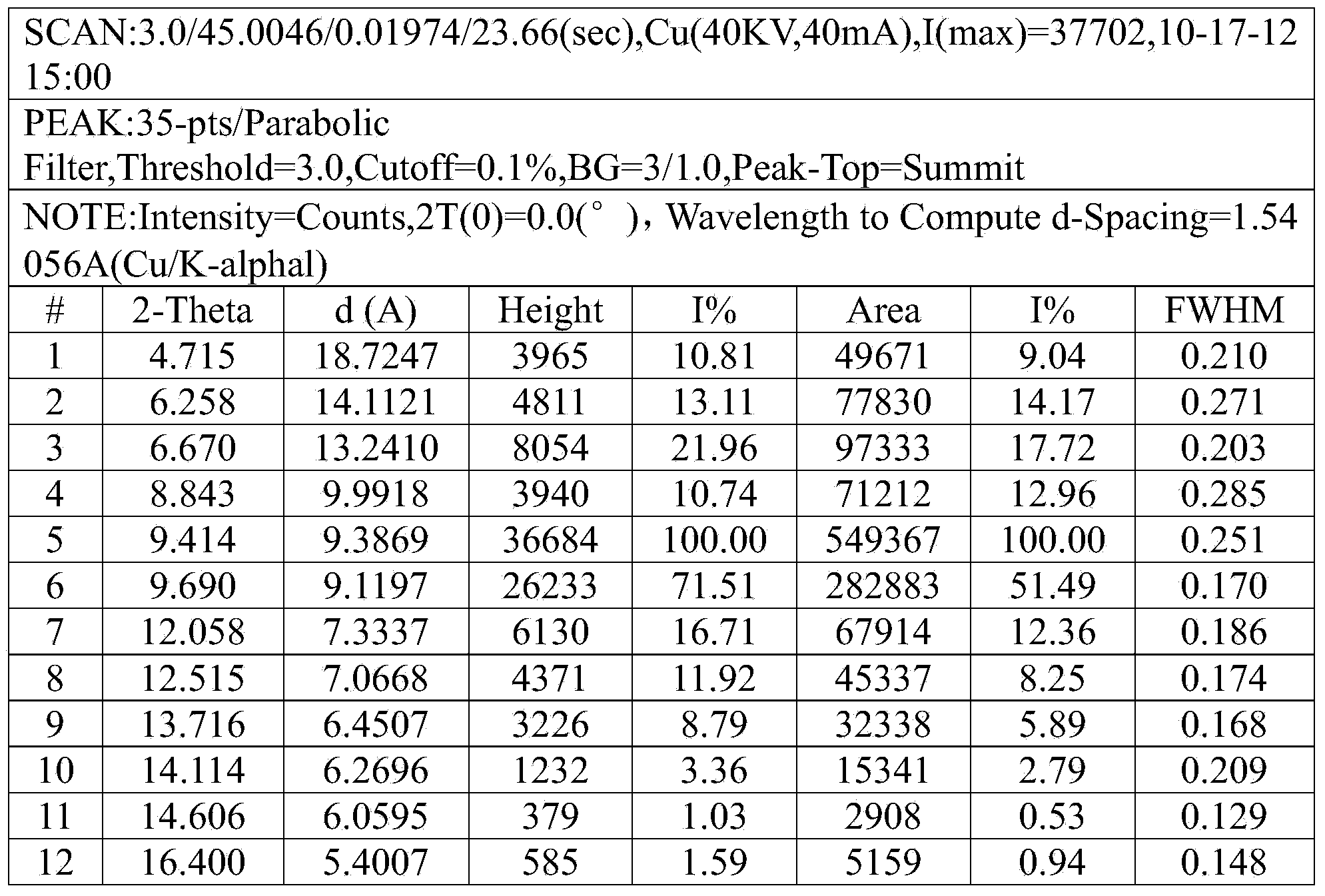
[0024] The resulting product X-ray powder diffraction pattern prepared in the present embodiment is as follows: figure 1 As shown, it can be seen from the figure that the X-ray powder diffraction spectrum of the ceftizoxime sodium crystal form of the present embodiment is expressed at 4.7 °, 6.3 °, 6.7 °, 8.8 °, 9.4 °, 9.7 ° with th...
Embodiment 2
[0029] The preparation method of a crystal form of ceftizoxime sodium compound in this example comprises the following steps: take 10 g of ceftizoxime sodium raw material, add 65 ml of pure water, stir at room temperature (90 rpm) until completely dissolved, and then add 1.0 g of ceftizoxime sodium Activated carbon is adsorbed and filtered, and the pH value is controlled to be 6.5; then 300ml of absolute ethanol is slowly added to the above container within 50 minutes, and it is completely precipitated at 20°C, and dried to constant weight by suction filtration. -0.03MPa, dried for 4.5h; 7.4g of fine granular ceftizoxime sodium compound crystal form was obtained. The X-ray powder diffraction pattern of the crystal form obtained is the same as in Example 1. When carrying out the stability test, the mass percentage content of related substances is 0.11%, and the mass percentage content of ceftizoxime polymer is 0.05%.
Embodiment 3
[0040] A pharmaceutical preparation for injection prepared by using the crystal form of ceftizoxime sodium compound prepared in Example 1 of this embodiment comprises the following steps: taking 1000 g of the crystal form of ceftizoxime sodium compound prepared in Example 1 (according to ceftizoxime sodium compound crystal form) In the local 100-level clean area, it is divided into 1000 bottles of 10ml molded antibiotic glass bottles, and then stoppered and capped to obtain ceftizoxime sodium for injection, the specification is: 1.0 (according to ceftizoxime ). The obtained product is a pharmaceutical preparation whose active ingredient is the crystal form of the ceftizoxime sodium compound (ie, the crystal form of ceftizoxime sodium).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com