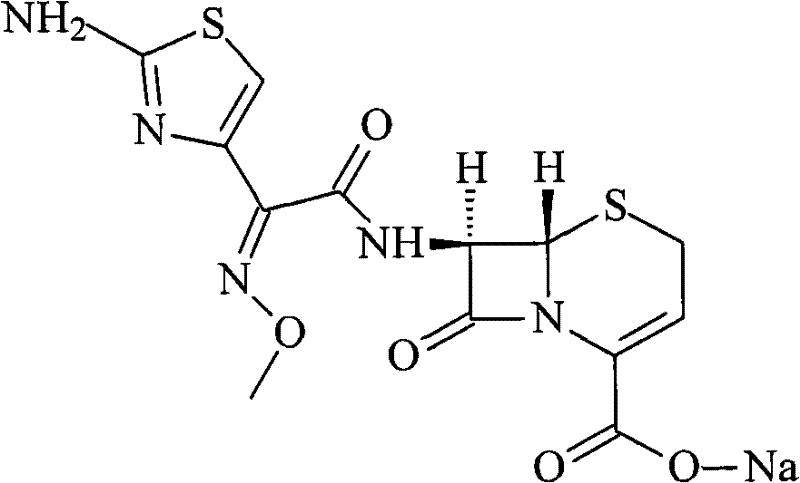
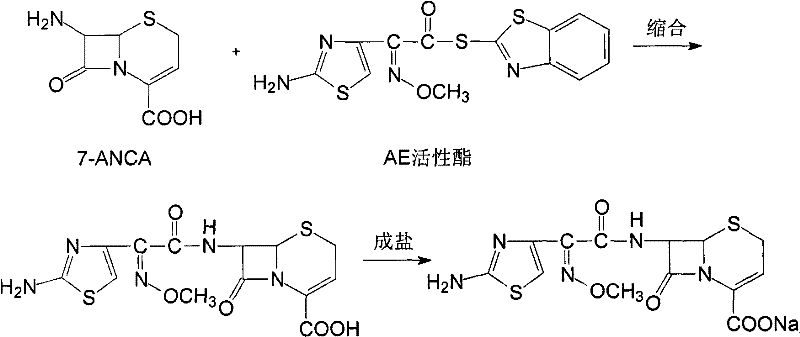
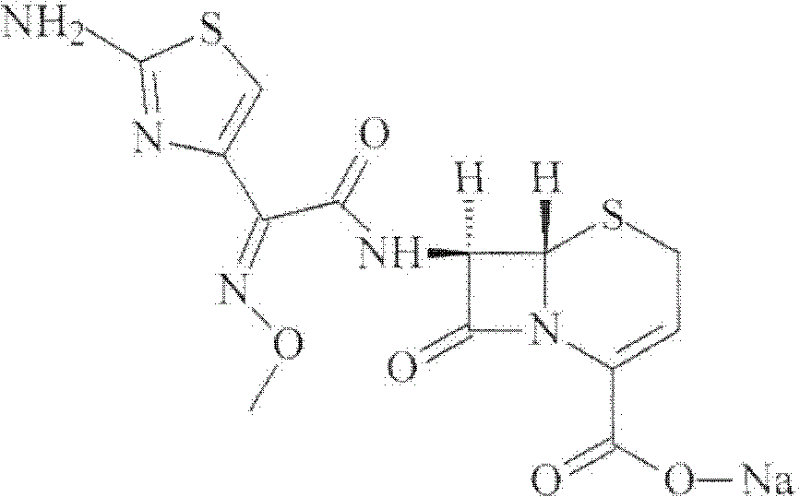
Cefuroxime sodium compound and new preparation method thereof
A technology of ceftizoxime sodium and ceftizoxime acid, which is applied in the field of medicine, can solve the problems of difficult high-purity and high-yield compounds, excessive consumption of activated carbon, and complicated methods, so as to achieve improved purity and content, low cost, and high-quality products. The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Purification of Ceftizoxime Sodium Compound
[0033] (1) Dissolve 100 g of ceftizoxime sodium crude product with a purity of 95.8% in 1000 ml of purified water, slowly add 3% acetic acid solution until the pH of the solution is 3, stir, leave to cool, produce precipitation, filter to obtain ceftizoxime acid ;
[0034] (2) Dissolve ceftizoxime acid in 800ml of isopropanol and isopropyl ether in a mixed solvent of 1:3, add 0.8g of activated carbon, stir at 50°C for 20min, filter for decarburization, and collect the filtrate;
[0035] (3) The filtrate obtained in the previous step is evaporated under reduced pressure, and the preparation chromatographic column is utilized to carry out separation and purification, and the mixed solvent of water and acetonitrile with a volume ratio of 1: 3 is the mobile phase, and the stationary phase filler is silica gel, and the flow rate is 2.8ml / min , the column temperature is 40°C; the fractions are collected in sections, ...
Embodiment 2
[0037] Example 2 Purification of Ceftizoxime Sodium Compound
[0038] (1) Dissolve 100 g of ceftizoxime sodium crude product with a purity of 95.8% in 1000 ml of purified water, slowly add 5% citric acid solution until the pH of the solution is 2.0, stir, leave to cool, produce precipitation, filter to obtain ceftizoxime acid;
[0039] (2) Dissolve ceftizoxime acid in 800ml of isopropanol and isopropyl ether 1:3 mixed solvent, add 2.4g of activated carbon, stir at 40°C for 30min, filter for decarburization, and collect the filtrate;
[0040] (3) The filtrate obtained in the previous step is evaporated under reduced pressure, and the preparation chromatographic column is utilized to carry out separation and purification, and the mixed solvent of water and acetonitrile with a volume ratio of 1: 3 is the mobile phase, and the stationary phase filler is silica gel, and the flow rate is 4.5ml / min , the column temperature is 30°C; the fractions are collected in sections, and th...
Embodiment 3
[0042] Example 3 Purification of Ceftizoxime Sodium Compound
[0043] (1) 100g of 95.8% pure ceftizoxime sodium crude product is dissolved in 1200ml of purified water, slowly add 7% oxalic acid solution until the pH of the solution is 4.0, stir, leave to cool, produce precipitation, filter to obtain ceftizole oxime acid;
[0044] (2) Dissolve ceftizoxime acid in 800ml of isopropanol and isopropyl ether in a 1:3 mixed solvent, add 1.6g of activated carbon, stir at 40°C for 20min, filter for decarburization, and collect the filtrate;
[0045] (3) The filtrate obtained in the previous step is evaporated under reduced pressure, and the preparation chromatographic column is utilized to carry out separation and purification, and the mixed solvent of water and acetonitrile with a volume ratio of 1: 3 is the mobile phase, and the stationary phase filler is silica gel, and the flow rate is 3.5ml / min , the column temperature is 30°C; the fractions are collected in sections, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com