Refining method of ceftizoxime sodium
A technology of cefizoxime sodium and a purification method, applied in the field of medicine, can solve the problems of many side reactions, low yield, complicated steps and the like, achieve high product yield and purity, improve total yield and purity, and reduce side reactions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
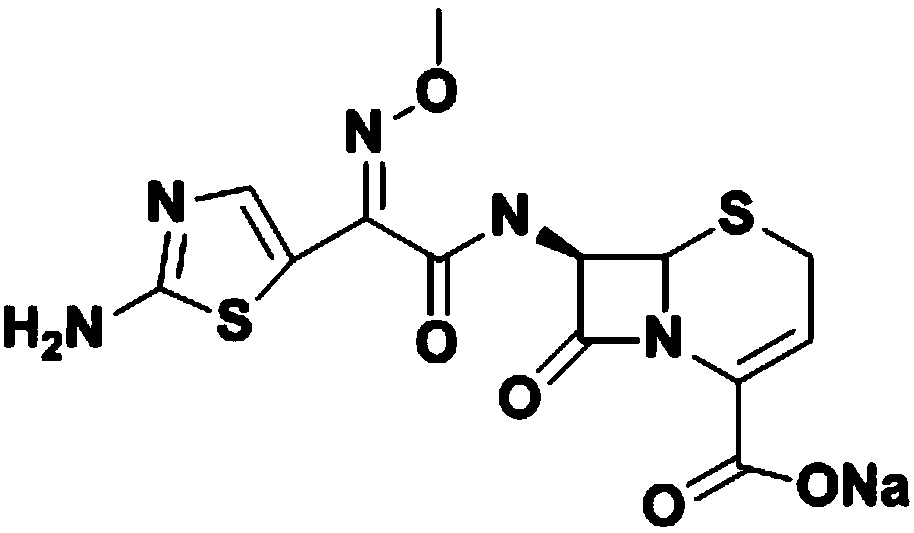
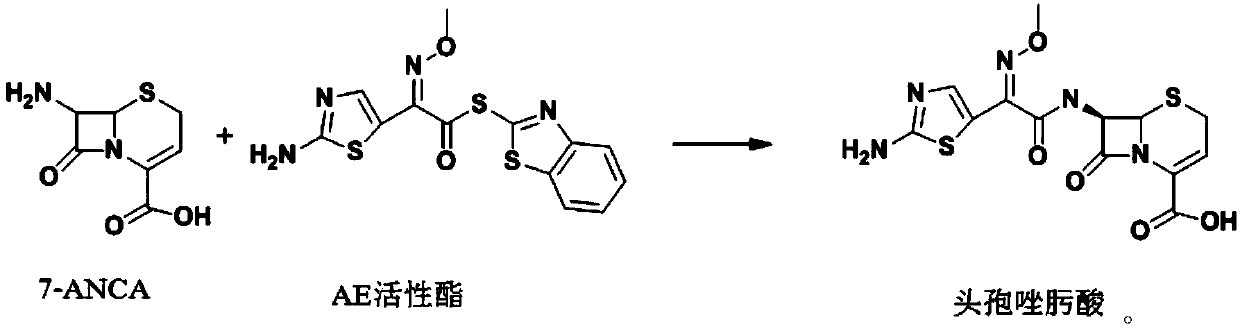
[0022] (1) Synthesis of ceftizoxamic acid
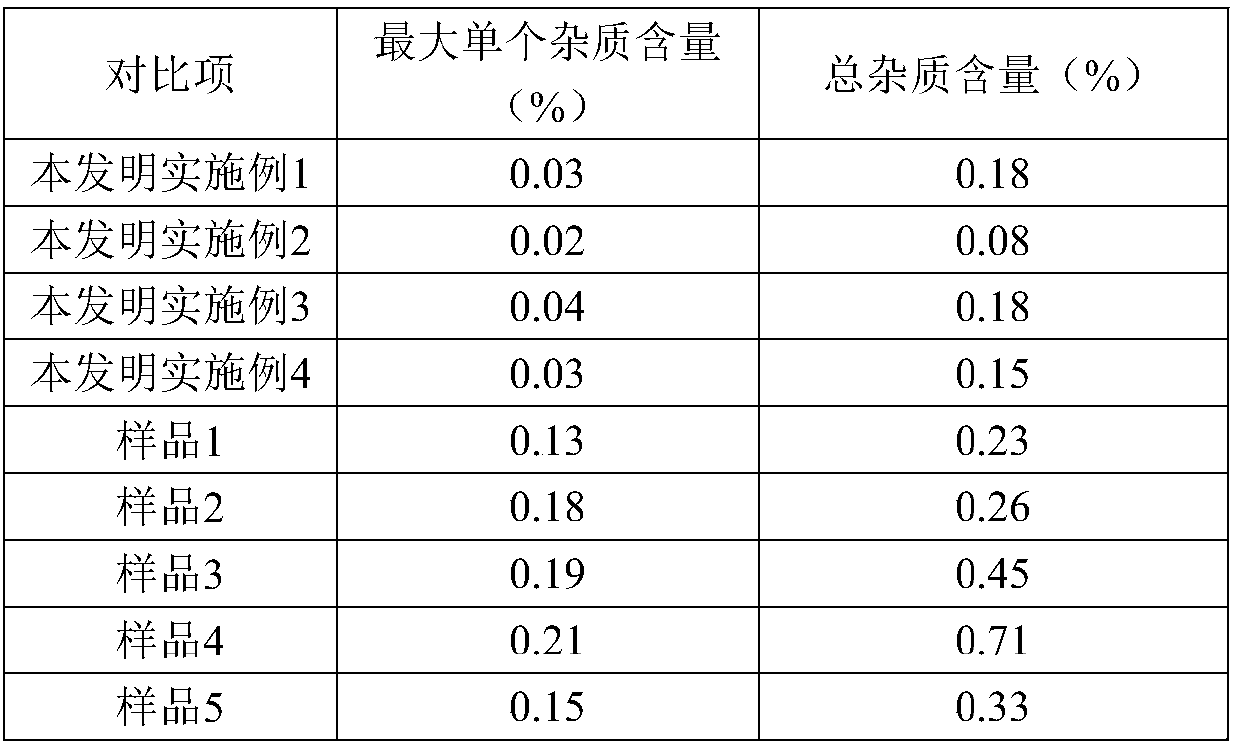
[0023] Add 20.01g (0.1mol) of 7-ANCA, 150ml of dichloromethane, and 0.15mol of N,N-diisopropylethylamine to the reactor in order to stir and dissolve, control the temperature at 0-5°C, and add AE active ester dropwise 35.02g (0.1mol), after 15 minutes, add 0.05mol of 4-dimethylaminopyridine, keep the temperature condition unchanged, stir and react for 3-4h, after the reaction, add water at room temperature for layering, and organic The phases are separated, the aqueous phase is retained, and dichloromethane is added for washing, then activated carbon is added for decolorization, hydrochloric acid is added for crystallization, centrifugation, and drying obtains 33.02 g of ceftizoxamic acid, the product yield is 86%, the purity is 99.5%, and the maximum single hetero 0.08%, total miscellaneous 0.52%.
[0024] (2) Refining of Ceftizoxime Sodium
[0025] Add 33.02 g of ceftizoxime acid to 198 ml of water to dissolve, add concentrated h...
Embodiment 2
[0027] (1) Synthesis of ceftizoxamic acid
[0028] Add 20.02g (0.1mol) of 7-ANCA, 150ml of dichloromethane, and 0.15mol of triethylamine to the reactor in order to stir and dissolve, and control it at -5-0°C, add 38.52g (0.11mol) of AE active ester dropwise , after 15 minutes, add 0.05 mol of 4-dimethylaminopyridine, keep the temperature constant, and stir for 3-4 hours. After the reaction, add water at room temperature for layering, separate the organic phase, and keep the water phase, adding dichloromethane for washing, then adding activated carbon for decolorization, adding hydrochloric acid for crystallization, centrifuging, and drying to obtain 36.79 g of ceftizoxamic acid, with a yield of 96%, a purity of 99.7%, a maximum of 0.05% of impurities, and a total of 0.22% of impurities.
[0029] (2) Refining of Ceftizoxime Sodium
[0030] Add 36.79g of ceftizoxime acid to 221ml of water to dissolve, add concentrated hydrochloric acid to control the pH to 2.5, add 16.13g (0.19...
Embodiment 3
[0032] (1) Synthesis of ceftizoxamic acid
[0033] Add 20.02g (0.1mol) of 7-ANCA, 150ml of dichloromethane, and 0.15mol of triethylamine to the reactor in order to stir and dissolve, and control the condition of 5-10°C, add 42.01g (0.12mol) of AE active ester dropwise, Add in 15 minutes, keep the temperature condition unchanged, stir and react for 3-4h, after the reaction is over, add water at room temperature for layering, separate the organic phase, keep the water phase, add dichloromethane for washing, and then add activated carbon Decolorize, add hydrochloric acid to crystallize, centrifuge, and dry to obtain 31.84 g of ceftizoxime acid, with a yield of 83%, a purity of 99.6%, a maximum of 0.15% of impurities, and 0.39% of total impurities.
[0034] (2) Refining of Ceftizoxime Sodium
[0035] Add 31.84g of ceftizoxime acid to 191ml of water to dissolve, add concentrated hydrochloric acid to control the pH to 2, add 13.94g (0.166mol) of sodium bicarbonate, add 64ml of meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com