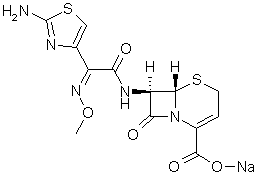
Preparation method of high purity Ceftizoxime
A ceftizoxime sodium, high-purity technology, applied in the field of medicine, can solve the problems of drug purity not meeting the requirements, improper production process control, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] Accurately weigh 10g of Ceftizoxime Sodium bulk drug with a purity of 95%. Add the solution containing ceftizoxime sodium into a fixed bed filled with D001 type strongly acidic styrene-based cation exchange resin, continue the exchange until the pH value is 3.5, and then use 10% hydrochloric acid aqueous solution as the eluent to elute, collect The eluate was concentrated under reduced pressure.
[0026] Then use 1M sodium carbonate aqueous solution to adjust the pH value to 7 when the temperature is raised to 65 degrees, and filter out the insoluble matter while it is hot.
[0027] Raise the temperature to 80°C, keep it for 30 minutes, add 120% volume of absolute ethanol, slowly lower the temperature, when the temperature drops to 10°C, crystals are precipitated, and after standing overnight, crystals are obtained, and after drying, 9.7 g of ceftizoxime sodium is obtained. The yield is 97%.
[0028] The high-performance liquid phase checks that the purity is 99.7%, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com