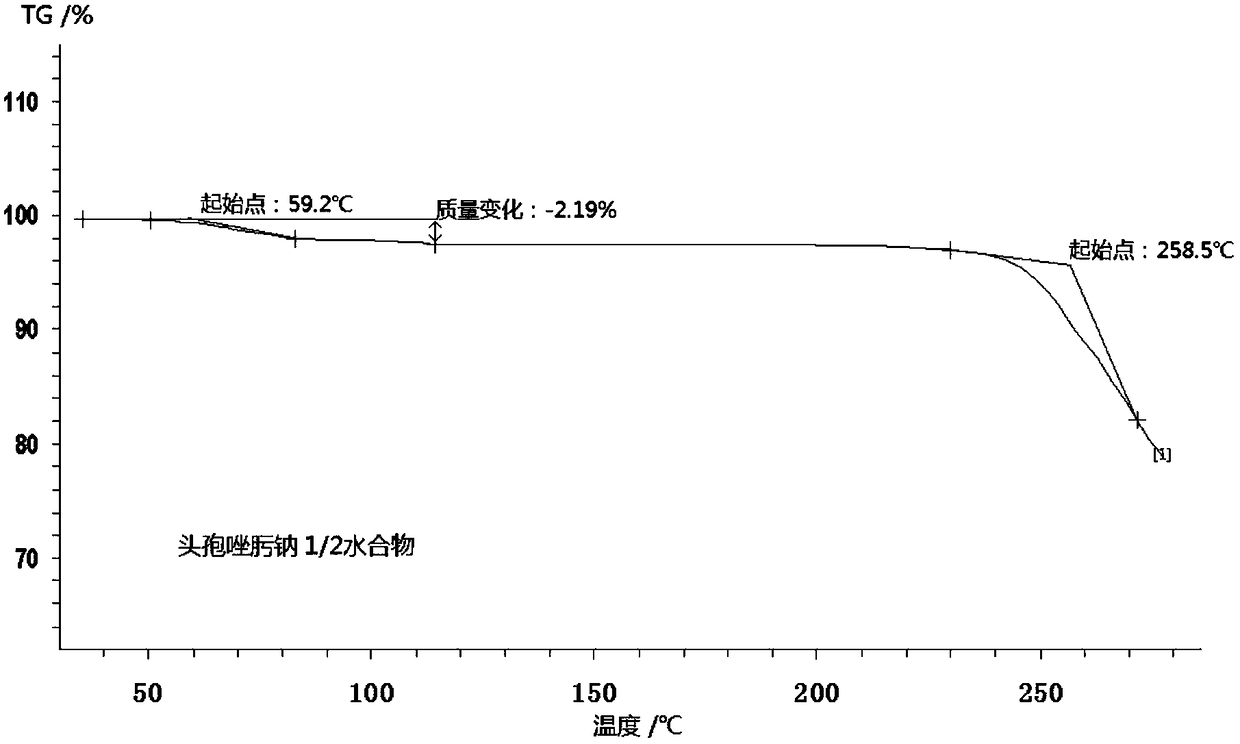
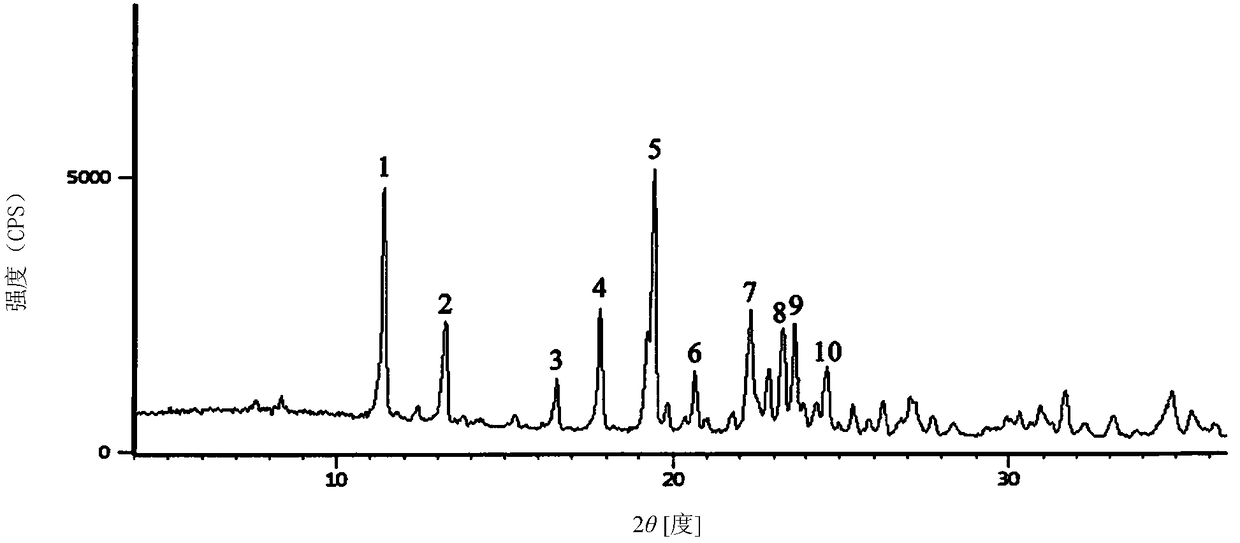
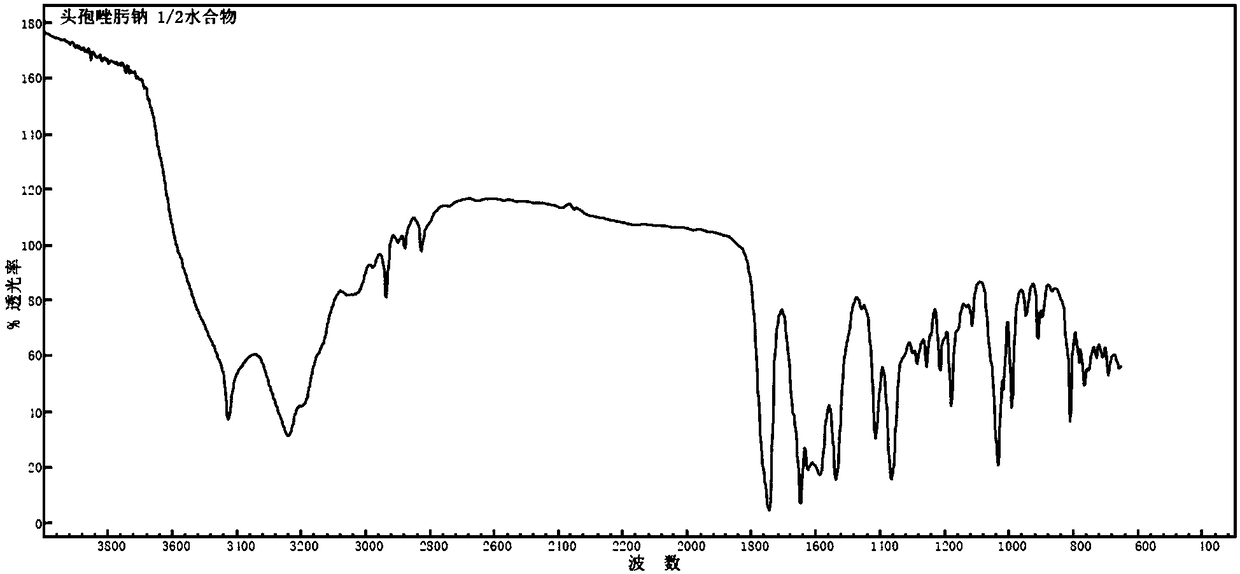
1/2 water ceftizoxime sodium compound
A technology for hydroceftizoxime and ceftizoxime sodium, which is applied in the field of chemical engineering and pharmaceutical crystallization, can solve the problems of complicated operation, many side reactions, poor fluidity and the like, and achieves wide application prospect, stable product quality and good thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]The preparation of embodiment 1 1 / 2 water ceftizoxime sodium compound
[0028] (1) Add 100g of 7-ANCA into 2L of chloroform, stir to form a suspension, slowly add 200ml of triethylamine, stir for 20min, then add 193g of AE-active ester and react at 30°C for 4h, after the reaction is complete, lower the temperature to 10°C , Add 4L of water for extraction, separate the water phase, add 20g of activated carbon, stir and absorb for 30min, filter, wash, combine the filtrate, adjust the pH value to 2.0 with 2mol / L hydrochloric acid solution, stir and crystallize at 10°C for 1h, filter, and wash with chloroform , dried under vacuum at 30°C for 20 minutes to obtain 169.3 g of ceftizoxime acid;
[0029] (2) Add ceftizoxime acid into 500ml of purified water, stir to form a suspension, lower the temperature to 5°C, slowly add 70ml of triethylamine, stir to dissolve, add 5g of activated carbon and stir for 20min to decolorize, filter, wash with purified water, slowly add Dissolve ...
Embodiment 2
[0033] The preparation of embodiment 2 1 / 2 water ceftizoxime sodium compound
[0034] (1) Add 120g of 7-ANCA into 2.5L of chloroform, stir to form a suspension, slowly add 240ml of triethylamine, stir for 20min, then add 232g of AE-active ester at 35°C for 5h, after the reaction is complete, lower the temperature to 15°C, add 5L of water for extraction, separate the water phase, add 20g of activated carbon, stir and adsorb for 30min, filter, wash, combine the filtrate, adjust the pH value to 2.5 with 2mol / L hydrochloric acid solution, stir and crystallize at 10°C for 1h, filter, trichloro Washed with methane, dried under vacuum at 30°C for 25 minutes to obtain 211.8 g of ceftizoxime acid;
[0035] (2) Add ceftizoxime acid into 500ml of purified water, stir to form a suspension, lower the temperature to 10°C, slowly add 100ml of triethylamine, stir to dissolve, add 5g of activated carbon and stir for 20min to decolorize, filter, wash with purified water, slowly add Dissolve 25...
Embodiment 3
[0039] Example 3 Preparation of 1 / 2 water ceftizoxime sodium compound
[0040] (1) Add 90g of 7-ANCA into 1.5L of chloroform, stir to form a suspension, slowly add 180ml of triethylamine, stir for 20min, then add 174g of AE-active ester and react at 35°C for 4.5h, after the reaction is complete, lower the temperature To 12°C, add 4L of water for extraction, separate the water phase, add 20g of activated carbon, stir and absorb for 20min, filter, wash, combine the filtrate, adjust the pH value to 2.2 with 2mol / L hydrochloric acid solution, stir and crystallize at 12°C for 1.5h, filter, Wash with chloroform, and dry under vacuum at 35°C for 20 minutes to obtain 152.4 g of ceftizoxamic acid;
[0041] (2) Add ceftizoxime acid into 400ml of purified water, stir to form a suspension, lower the temperature to 8°C, slowly add 90ml of triethylamine, stir to dissolve, add 5g of activated carbon and stir for 20min to decolorize, filter, wash with purified water, slowly add Dissolve 200m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com