Cyclobenzaprine hydrochloride sustained-release capsule and preparation method thereof
A technology of cyclobenzaprine hydrochloride and sustained-release capsules, which is applied in the direction of microcapsules, capsule delivery, and pharmaceutical formulations, and can solve the problems of short half-life, poor compliance, and slow elimination of cyclobenzaprine hydrochloride, and prolong the effective time of action , stable quality, uniform and slow drug release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
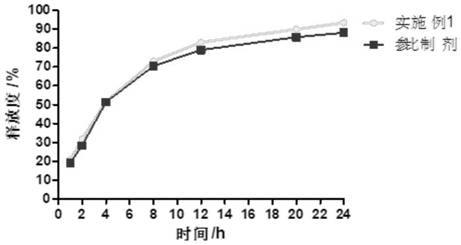
[0029] Embodiment 1 A kind of cyclobenzaprine hydrochloride sustained-release capsule and preparation method thereof
[0030] The cyclobenzaprine hydrochloride was pulverized using a pulverizer and passed through a 100-mesh sieve; 30g of cyclobenzaprine hydrochloride, 20g of hydroxypropyl methylcellulose E5, and 90g of microcrystalline cellulose PH101 were weighed and added to the wet granulator and mixed Evenly, add an appropriate amount of aqueous solution to obtain a suitable soft material, the stirring speed is 400 rpm, and the cutting speed is 400 rpm; the soft material is extruded and spheronized to obtain wet skeleton pellets, the extrusion speed is 30 rpm, the spheronization speed is 1000 pm, and the spheronization time is 5 minutes to obtain wet skeleton pellets. Pill core; place the wet pill in a fluidized bed to dry for 10 minutes, sieve to obtain a 20-40 mesh pill core; weigh 10g Eudragit RS100, 2g triethyl citrate, 2g povidone k30, 5g stearic acid Magnesium is dis...
Embodiment 2
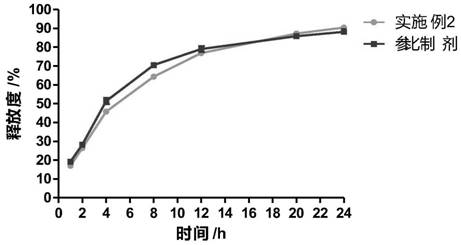
[0031] Embodiment 2 A kind of cyclobenzaprine hydrochloride sustained-release capsule and preparation method thereof
[0032] The cyclobenzaprine hydrochloride was pulverized using a pulverizer and passed through a 100-mesh sieve; 30g of cyclobenzaprine hydrochloride, 20g of hydroxypropyl methylcellulose E5, and 90g of microcrystalline cellulose PH101 were weighed and added to the wet granulator and mixed Evenly, add an appropriate amount of aqueous solution to obtain a suitable soft material, the stirring speed is 400 rpm, and the cutting speed is 400 rpm; the soft material is extruded and spheronized to obtain wet skeleton pellets, the extrusion speed is 30 rpm, the spheronization speed is 1000 pm, and the spheronization time is 5 minutes to obtain wet skeleton pellets. Pill core; place the wet pill in a fluidized bed to dry for 10 minutes, sieve to obtain a 20-40 mesh pill core; weigh 10g Eudragit NE30D, 2g triethyl citrate, 2g povidone k30, 5g stearin Magnesium acid is dis...
Embodiment 2
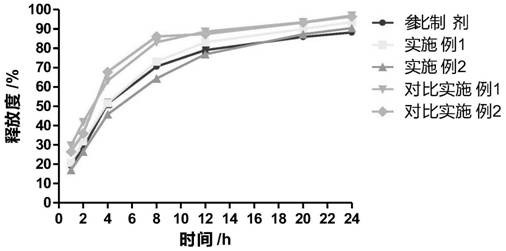
[0035] Comparative Example 2 Single film-controlled sustained-release capsule and preparation method thereof
[0036]The cyclobenzaprine hydrochloride was pulverized by a pulverizer, and passed through a 100-mesh sieve; 30 g of cyclobenzaprine hydrochloride and 110 g of microcrystalline cellulose PH101 were weighed into a wet granulator and mixed evenly, and then an appropriate amount of aqueous solution was added to prepare a suitable soft granulator. material, stirring speed 400rpm, chopping speed 400rpm; extruding and spheronizing the soft material to obtain wet skeleton pills, extrusion speed 30rpm, spheronization speed 1000pm, spheronization time 5 minutes, to obtain wet pill cores; put the wet pills in a fluidized Dry in the bed for 10 minutes, sieve to obtain 20-40 mesh pill cores; weigh 10g Eudragit RS100, 2g triethyl citrate, 2g povidone k30, 5g magnesium stearate and disperse them in 90% ethanol, stir evenly , the preparation concentration is 5% coating liquid; the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com