Preparation of controlled release skeletal muscle relaxant dosage forms
a technology of skeletal muscle relaxant and oral dosage form, which is applied in the direction of biocide, muscular disorder, drug composition, etc., can solve the problem of patient compliance with oral administration of thrice daily
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
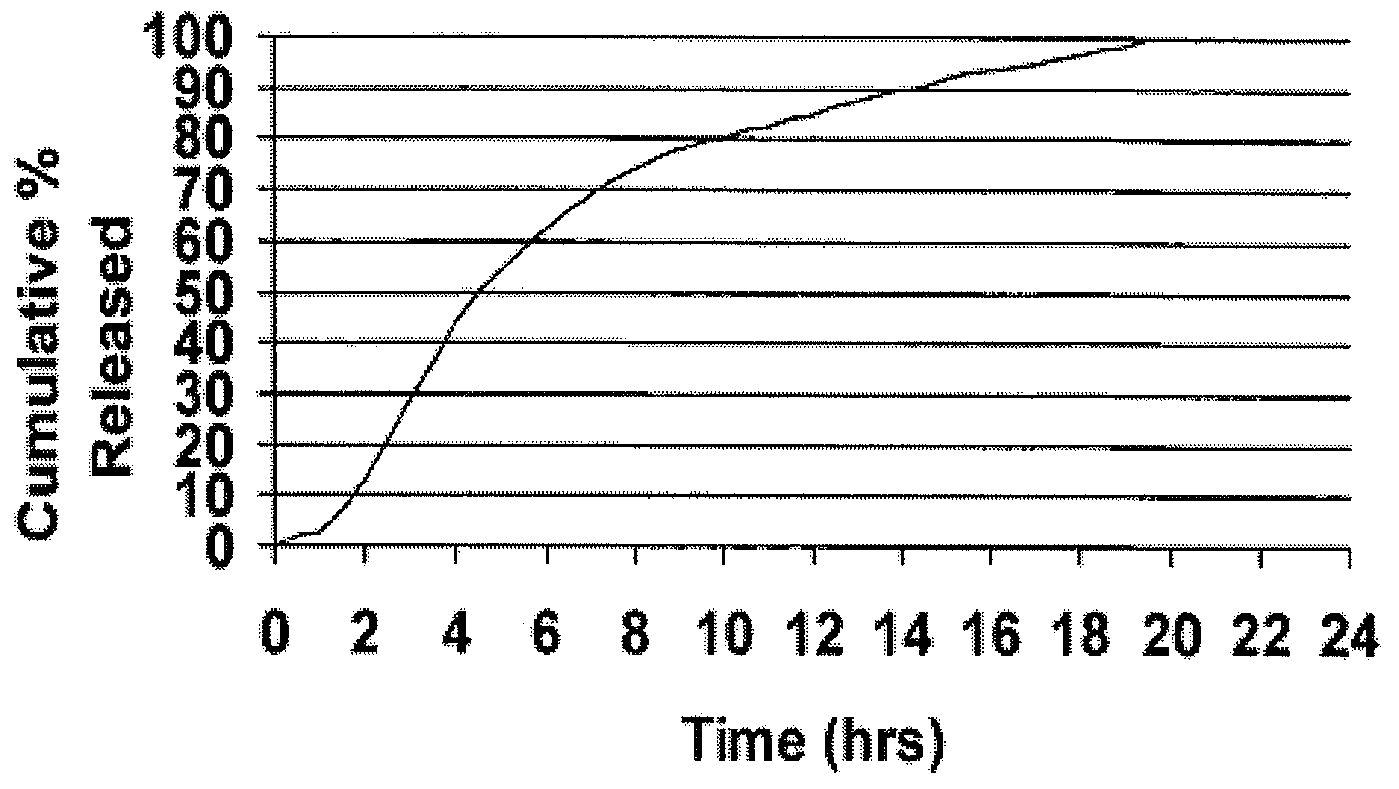
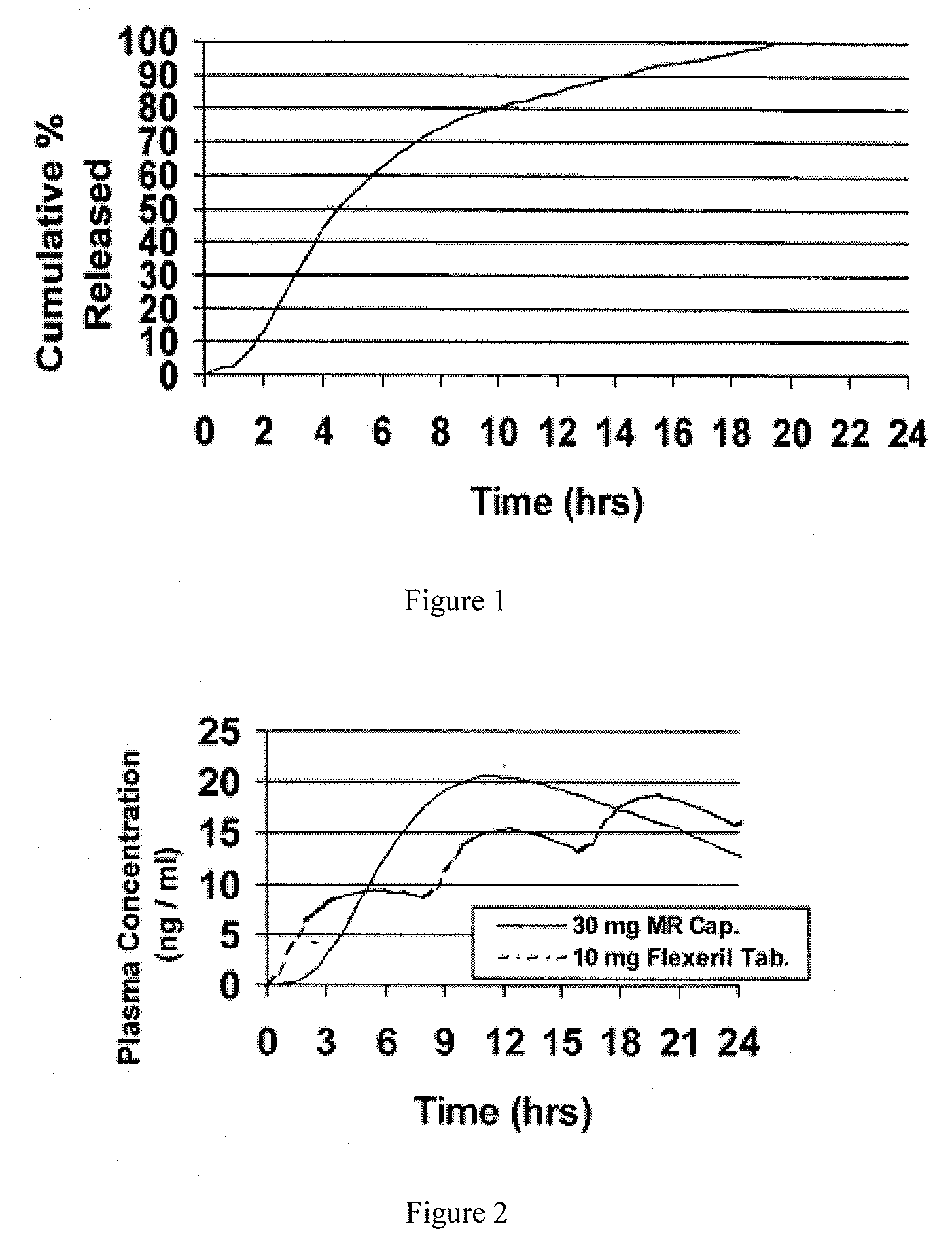
[0089]Cyclobenzaprine is well absorbed after oral administration, but there is a large intersubject variation in plasma levels. It is eliminated quite slowly with a half-life as long as one to three days. The present treatment regimen of 10 mg three times daily is an issue of patient compliance, especially the elderly. Hence, a modified release dosage form (capsule) was designed with a release profile shown in FIG. 1. To determine if this is the proper release profile, the pharmacokinetics data of cyclobenzaprine following a single dose of 10 mg Flexeril® tablets administered 3 times a day was taken from the literature. A pharmacokinetic model was developed from this data using WinNonlin™ Version 1.5.
[0090]The resulting model parameters are listed below:
Model ParameterValueVolume of Distribution / F429 LK010.2031 hr−1K100.1004 hr−1K120.0828 hr−1K210.0398 hr−1Tlag0 hrDose2 × 10 mg Tablets
[0091]Theoretical plasma levels were simulated using the pharmacokinetic model given above and the ...
example 2
[0092]A drug solution (25 wt. % solids) comprising cyclobenzaprine hydrochloride (1875.5 g) prepared in 50 / 50 acetone / purified water (2812.5 g each) and coated onto 20-25 mesh sugar spheres (5475 g) in a Glatt fluid bed coater, GPCG 5, equipped with a 9″ bottom spray Wurster type “B” insert (14″ high), a partition height from the distribution plate of about 53 mm, at the following conditions: Nozzle diameter: 1.0 mm; Room humidity: 64% RH; Atomization air pressure: 2.0-2.5 bar; Initial spray rate: 7 mL / min ramping up to about 60 mL / min; Product temperature: 50±1° C.; Process air volume: about 150 CFM. After drying at about 50° C. for 5 min, the resulting drug layered beads were provided with a protective seal coat of OPADRY® Clear at a coating level of 2 wt. % in the Glatt fluid bed coater by spraying the aqueous solution (10 wt. % solids) at a spray rate of about 8-10 g / min at a product temperature of 44° C., then dried at about 42° C. for 90 min to provide “immediate release” (IR)...
example 3
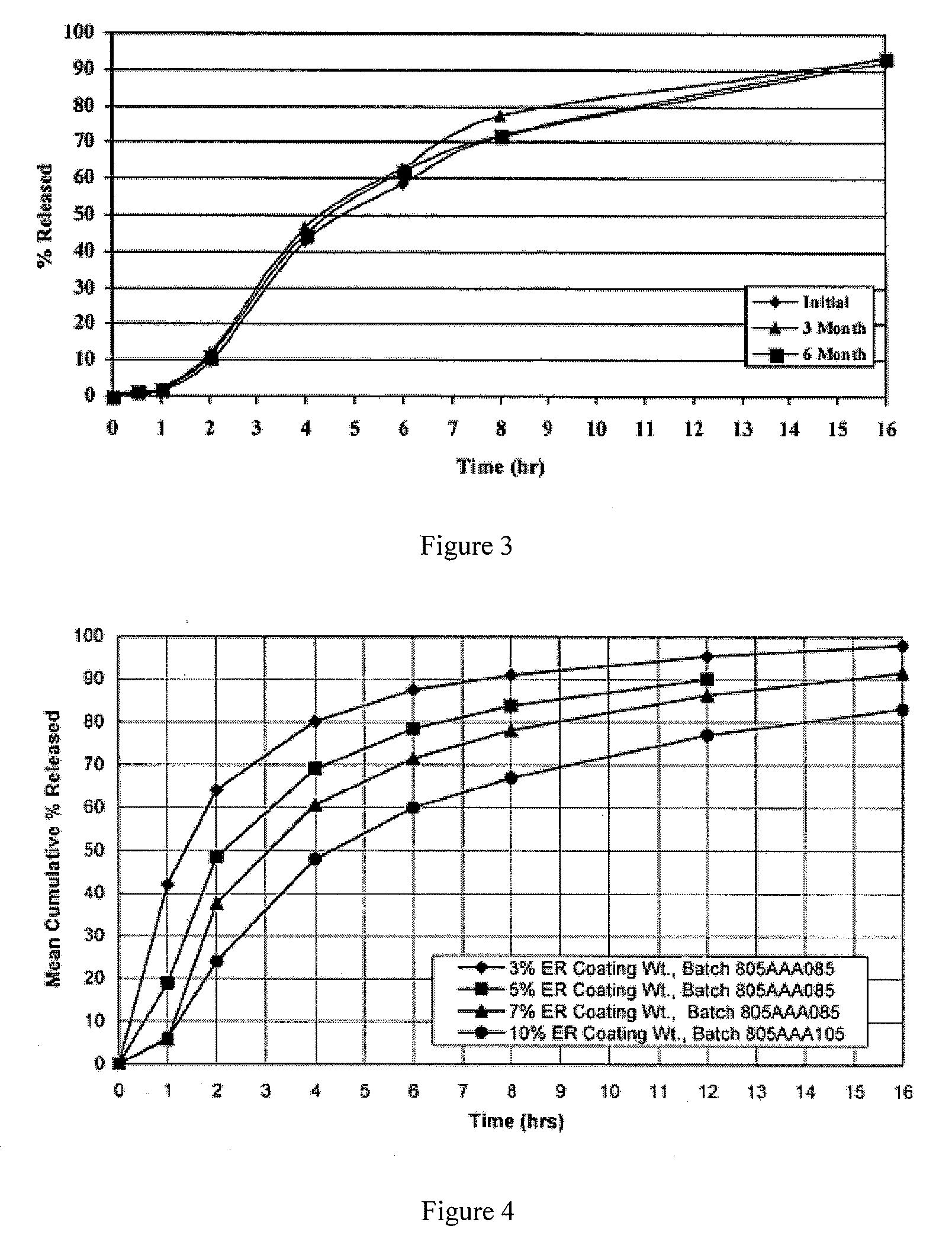
[0094]Cyclobenzaprine Hydrochloride (1,200 g) was slowly added to an aqueous solution of polyvinylpyrrolidone such as Povidone USP (K-29 / 32, 80 g) and mixed well. # 20-25 mesh sugar spheres (2,640 g) were coated with the drug solution in a Glatt fluid bed coater, equipped with a 9″ bottom spray Wurster insert to provide IR beads with a coating weight of about 9%. The drug containing particles were dried, and a seal coat of OPADRY® Clear (2% w / w) was first applied and dried in the Glatt fluid bed unit as a precautionary measure to drive off excessive surface moisture. The composition and batch quantities of the IR Beads were given in 5 to 10 kg. Following the second coating process the IR Beads were passed through 14 and 24 mesh screens. Beads remaining on the 14-mesh screen were discarded as oversized beads and beads passing through the 24-mesh screen were discarded as undersized beads.
[0095]The next step in the process was to apply an extended release polymer membrane by spraying A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dew point | aaaaa | aaaaa |
| dew point | aaaaa | aaaaa |
| dew point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com