Cyclobenzaprine hydrochloride sustained-release capsule and preparation method thereof
A technology of cyclobenzaprine hydrochloride and sustained-release capsules, which is applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as poor environmental friendliness, cumbersome process, and long time-consuming preparation process, and achieve reduction The effect of simple production cost and preparation process
Active Publication Date: 2022-05-27
NOVAST LABORATORIES (CHINA) LTD
View PDF7 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The prescription of the above-mentioned sustained-release capsules is complicated, the process is cumbersome, the preparation process takes a long time, the parameters are controlled too much, and the process stability is greatly affected by the performance of the equipment.
In addition, the sustained-release capsules need to use a large amount
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Login to View More
Abstract
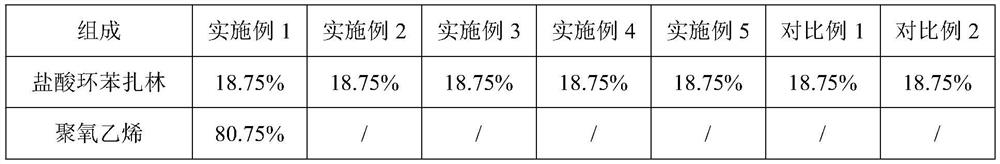
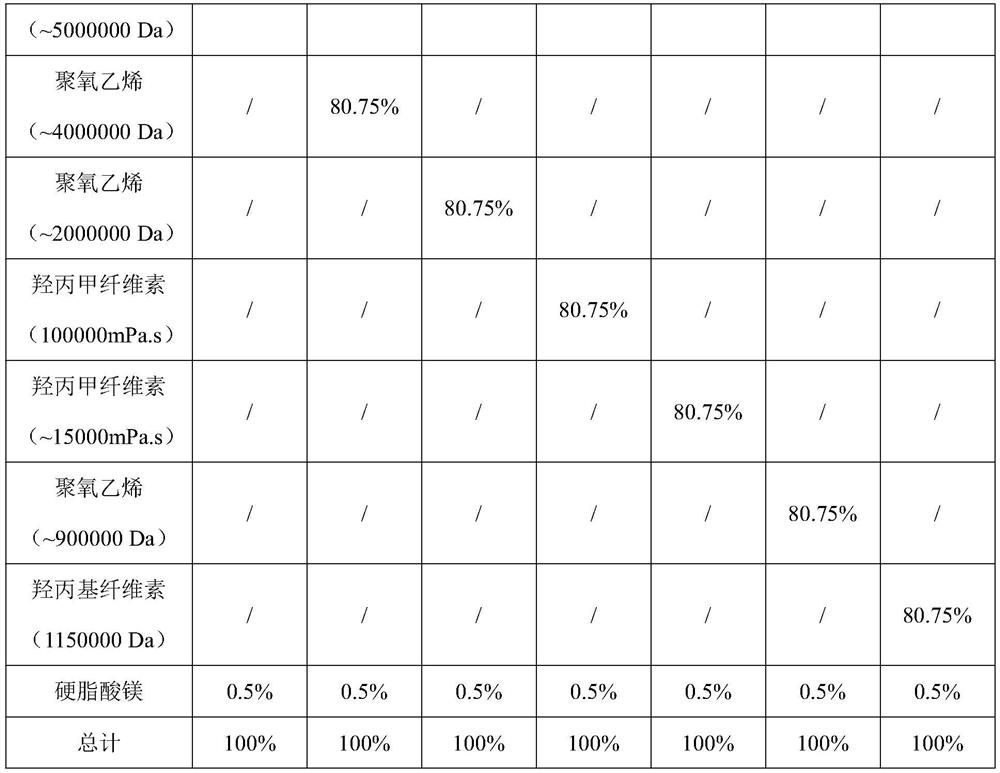
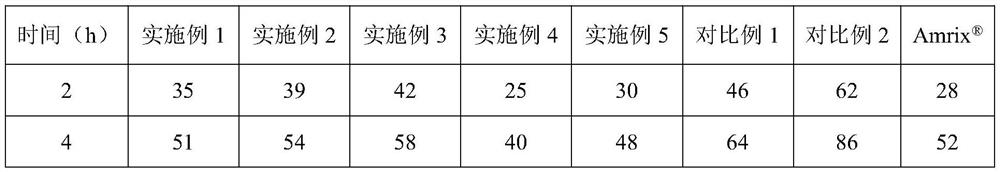
The invention relates to a cyclobenzaprine hydrochloride sustained-release capsule, the content of the cyclobenzaprine hydrochloride sustained-release capsule is a single cylinder formed by pressing mixed powder containing cyclobenzaprine hydrochloride, a sustained-release material and a lubricant, and the sustained-release material is selected from one or more of polyoxyethylene and hydroxypropyl methylcellulose. The cyclobenzaprine hydrochloride sustained-release capsule with an in-vitro dissolution behavior similar to that of a commercially available sustained-release pellet capsule is obtained by optimizing a sustained-release material of the capsule content. The preparation process of the preparation is simple, the quality is controllable, and the preparation is suitable for large-scale industrial production.
Description
technical field [0001] The invention belongs to the field of medicines, in particular to a cyclobenzaprine hydrochloride sustained-release capsule. Background technique [0002] The chemical name of cyclobenzaprine hydrochloride is 5-(3-dimethylaminopropene) dibenzo[a,e] cycloheptatriene hydrochloride, which is a tricyclic muscle relaxant developed by Merck , which was approved for marketing in more than ten countries including the United States in the 1980s, is mainly used clinically to treat musculoskeletal discomfort or muscle spasms accompanied by pain symptoms. The drug can significantly relieve muscle spasms and relieve pain, tenderness, Associated symptoms such as exercise restriction and restriction of daily activities. The results of animal experiments show that cyclobenzaprine hydrochloride can not directly interfere with animal muscle nerve function or directly affect skeletal muscle function, and it mainly acts on the brain part of the central nervous system, ra...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/52A61K31/135A61K47/38A61K47/34A61P21/02A61P29/00
CPCA61K31/135A61K9/4866A61P21/02A61P29/00A61K9/4833A61K9/4808Y02A50/30
Inventor 徐彦马甜甜朱硕然
Owner NOVAST LABORATORIES (CHINA) LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com