Cyclobenzaprine capsule
A technology for cyclobenzaprine and benzalkonium is applied in the field of medicine and achieves the effects of simple preparation process and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A preparation method of cyclobenzaprine enteric-coated capsules, comprising the following steps:
[0025] (1) Get cyclobenzaprine and filler to mix evenly, take 85% ethanol as binder, make 20-30 object cyclobenzaprine pellets, for subsequent use;
[0026] (2) Dissolve enteric-coated material, plasticizer and lubricant with 80% ethanol, make enteric-coated liquid;
[0027] (3) Evenly spray the enteric coating solution prepared in step (2) on the surface of the cyclobenzaprine pellets prepared in step (1), and obtain cyclobenzaprine enteric-coated pellets after drying;
[0028] (4) Filling cyclobenzaprine enteric-coated pellets into capsule shells to obtain cyclobenzaprine enteric-coated capsules.
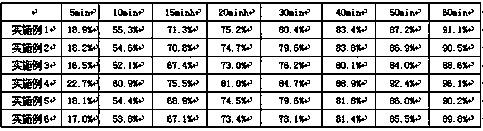
Embodiment 1-6
[0029] Example 1-6 Preparation of Cyclobenzaprine Enteric-Coated Capsules
[0030] Cyclobenzaprine enteric-coated capsules were prepared according to the raw and auxiliary materials in the following prescription and the above-mentioned preparation method. Among them, " / " means not used.
[0031]
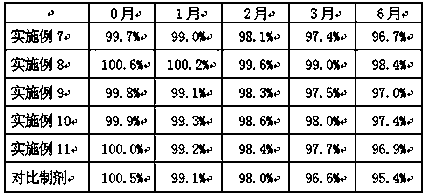
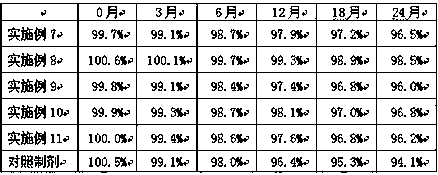
Embodiment 7-11
[0040] Example 7-11 Preparation of cyclobenzaprine enteric-coated capsules
[0041] Cyclobenzaprine enteric-coated capsules were prepared according to the raw and auxiliary materials in the following prescription and the above-mentioned preparation method.
[0042]
[0043] Test Example 2 Determination of Dissolution of Cyclobenzaprine Enteric-Coated Capsules Gained in Examples 7-11
[0044] The measurement method is the same as that of Test Example 1, and the measurement results are shown in Table 3 and Table 4.
[0045] Table 3 Dissolution of cyclobenzaprine enteric-coated capsules obtained in Examples 7-11 in artificial gastric juice
[0046]
[0047] As can be seen from Table 3, the cyclobenzaprine enteric-coated capsule of embodiment 8 dissolves the slowest in the pH1.2 artificial gastric juice, indicating that when the weight of the enteric-coated material methacrylic acid copolymer and hydroxypropyl methylcellulose phthalate When the ratio is 2:1, the prepared c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com