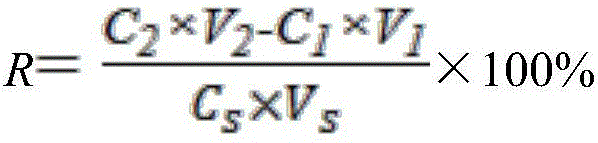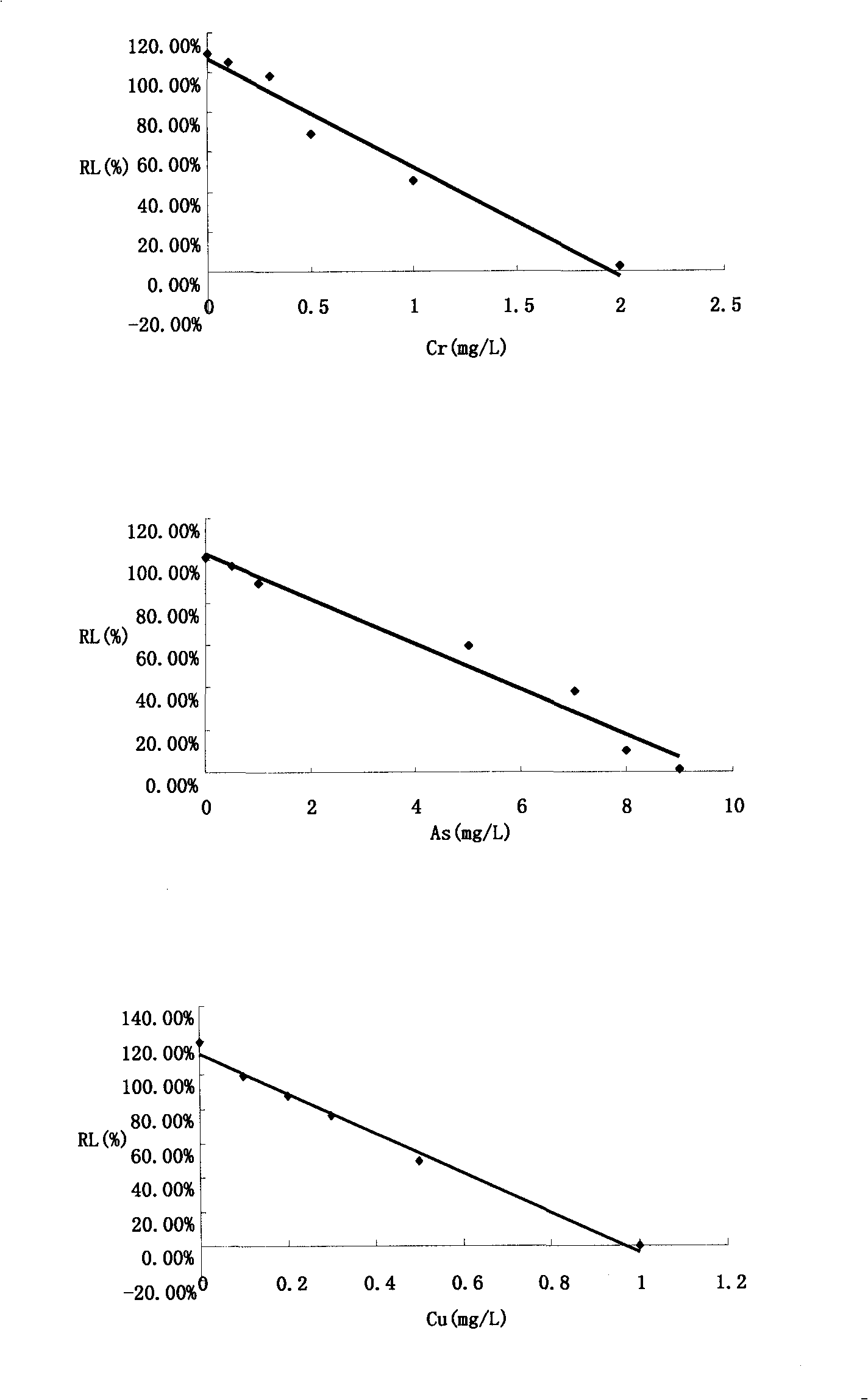Patents
Literature
117 results about "EC50" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Half maximal effective concentration (EC₅₀) refers to the concentration of a drug, antibody or toxicant which induces a response halfway between the baseline and maximum after a specified exposure time. EC₅₀ is also written as [A]₅₀. It is commonly used as a measure of a drug's potency, and the use of EC₅₀ is preferred over that of 'potency', which has been criticised for its vagueness. EC₅₀ is a measure of concentration, expressed in molar units (M), where 1 M is equivalent to 1 mol/L.
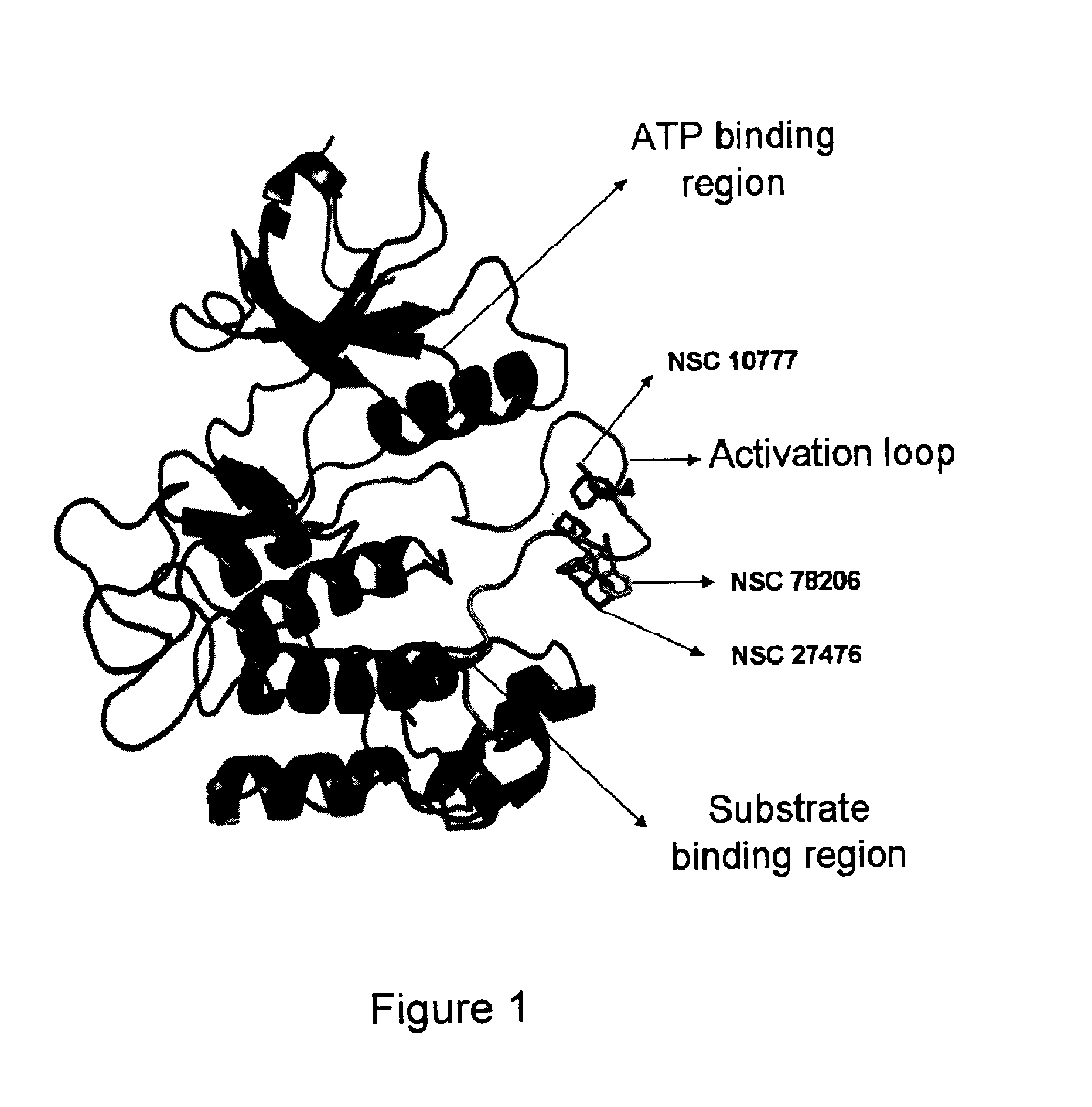
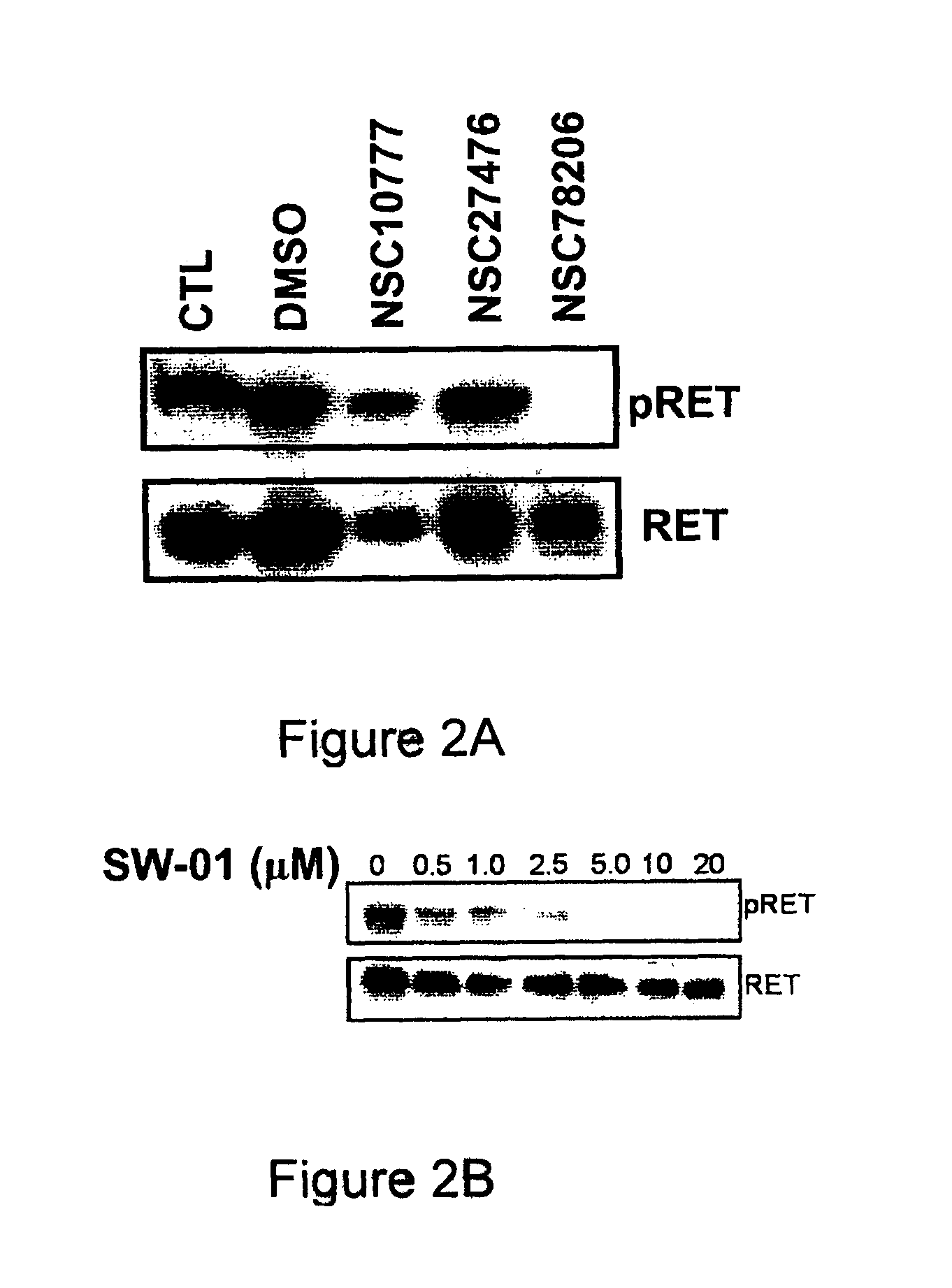
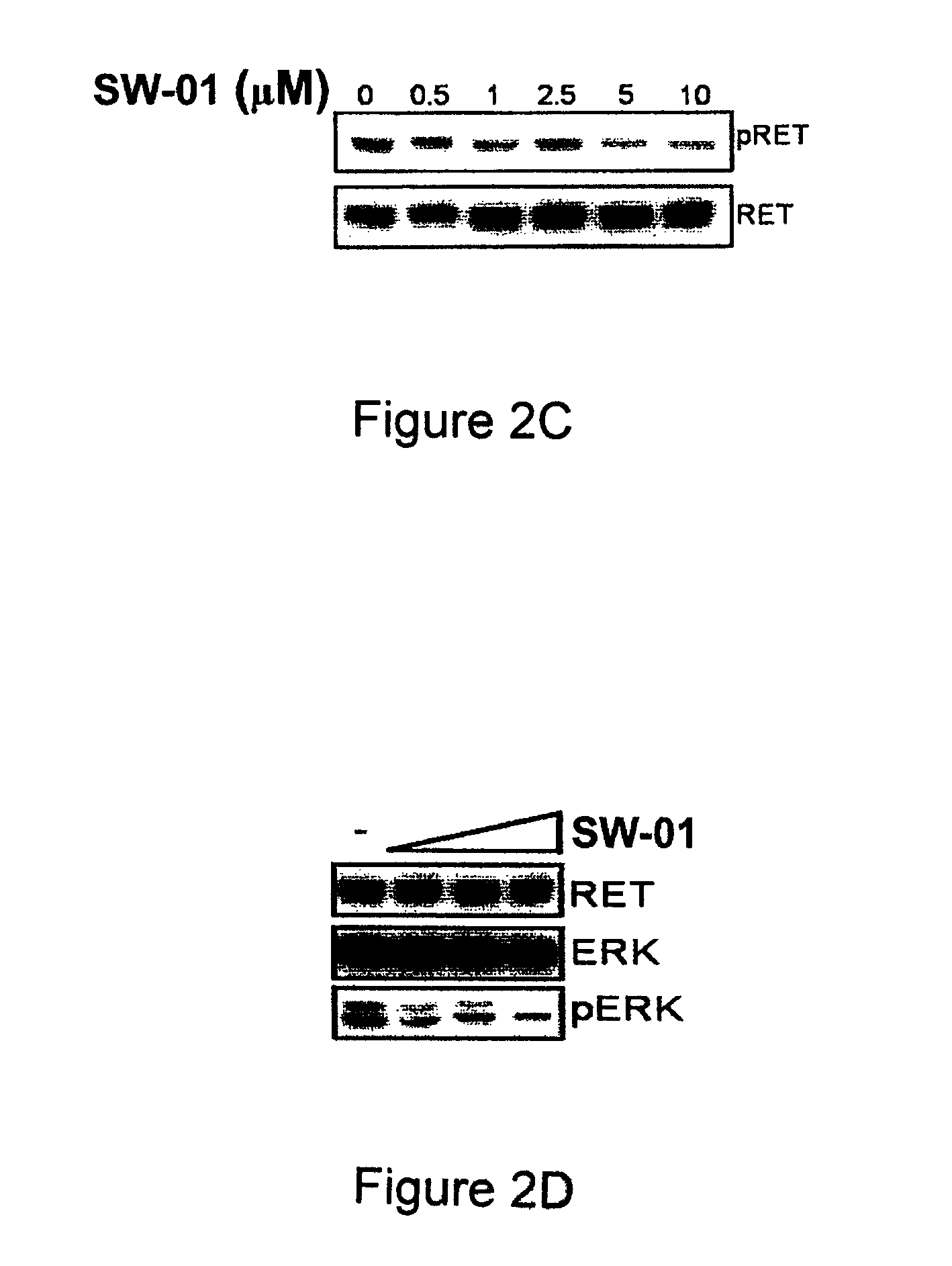
Pharmaceutical compositions comprising RET inhibitors and methods for the treatment of cancer
InactiveUS8629135B2Reduce transferGrowth inhibitionBiocideOrganic compound preparationCancer preventionAutophosphorylation
Owner:SINGH VINAY K +2
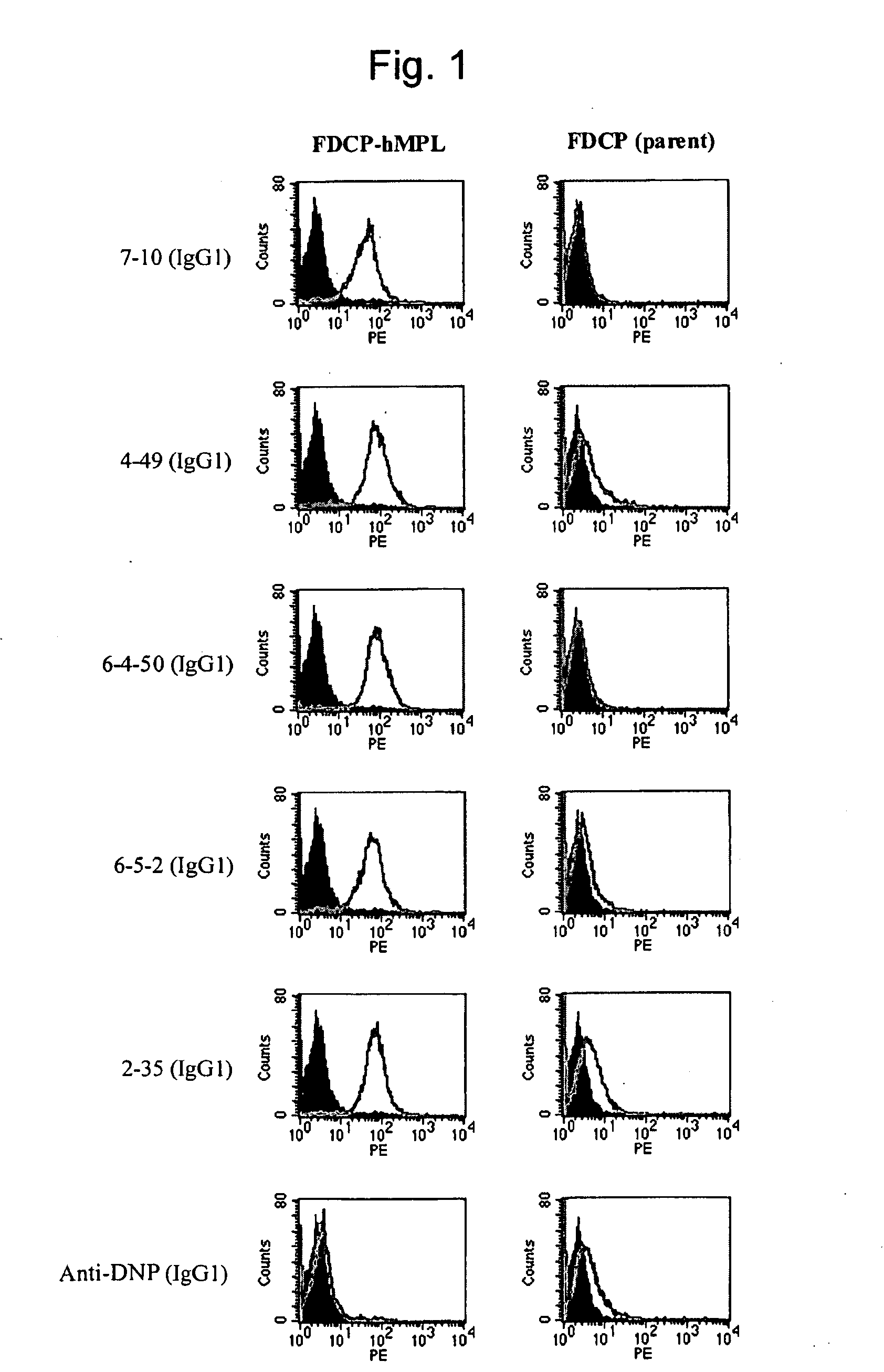
Agonist antibody to human thrombopoietin receptor
InactiveUS20100004429A1High activityLow antigenicityThrombopoietinHybrid immunoglobulinsHuman plateletUmbilical cord
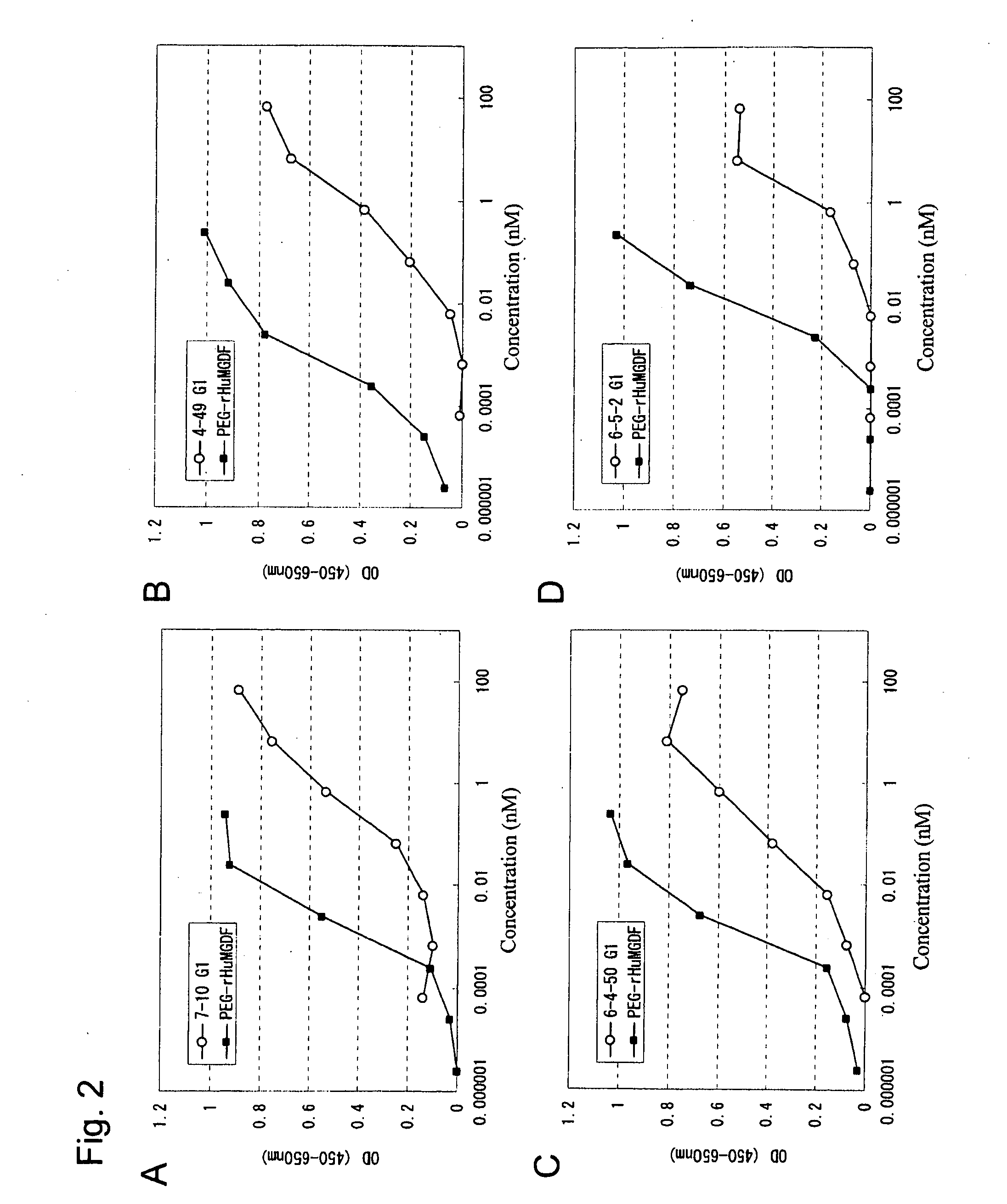
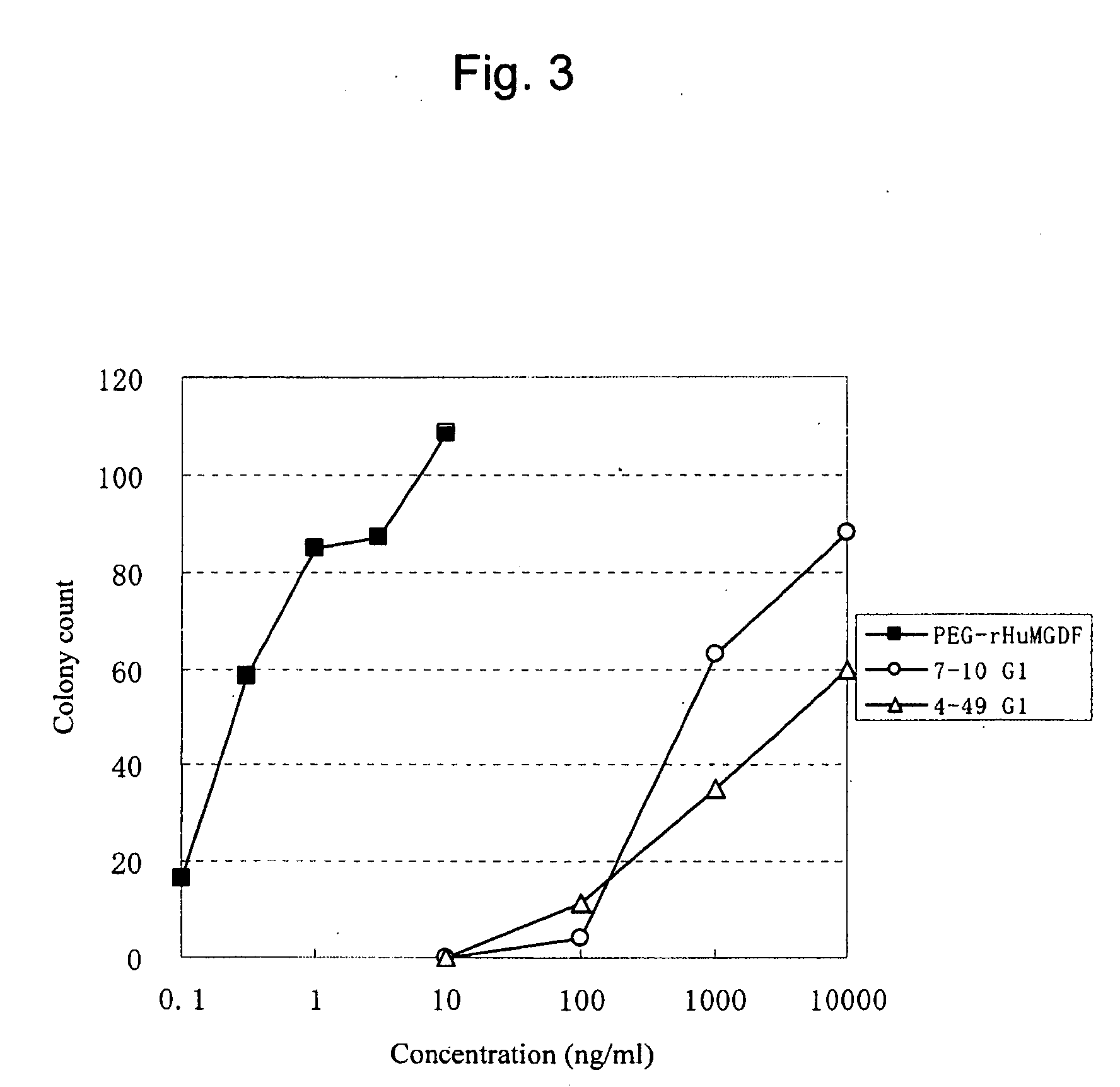
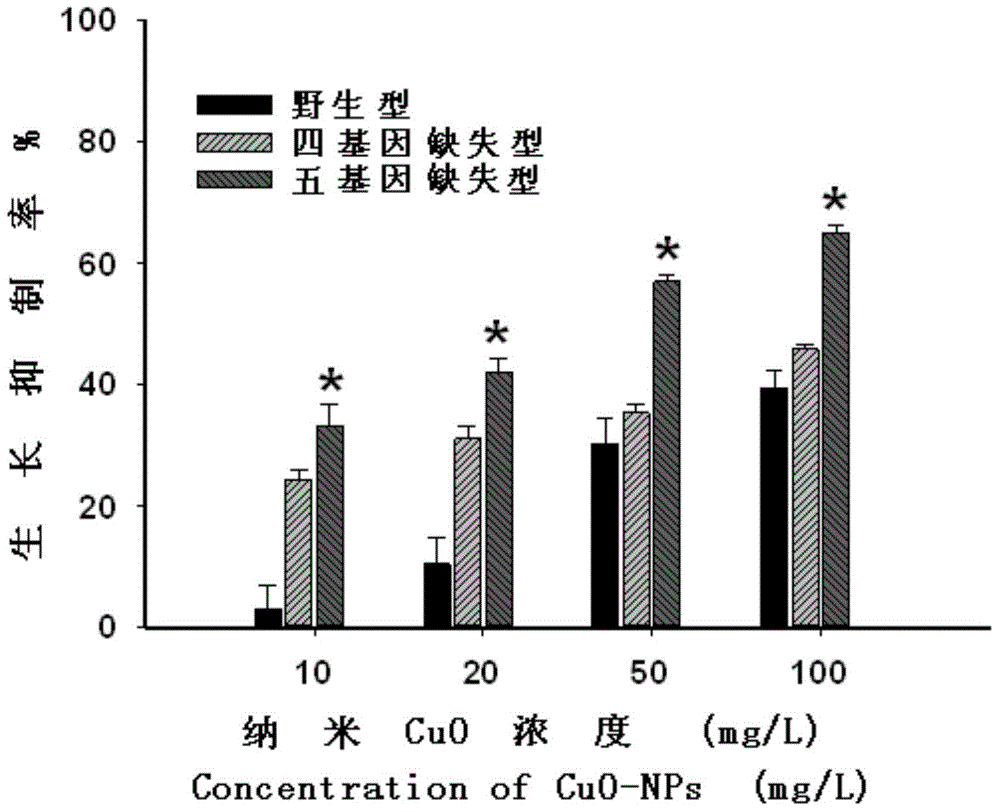
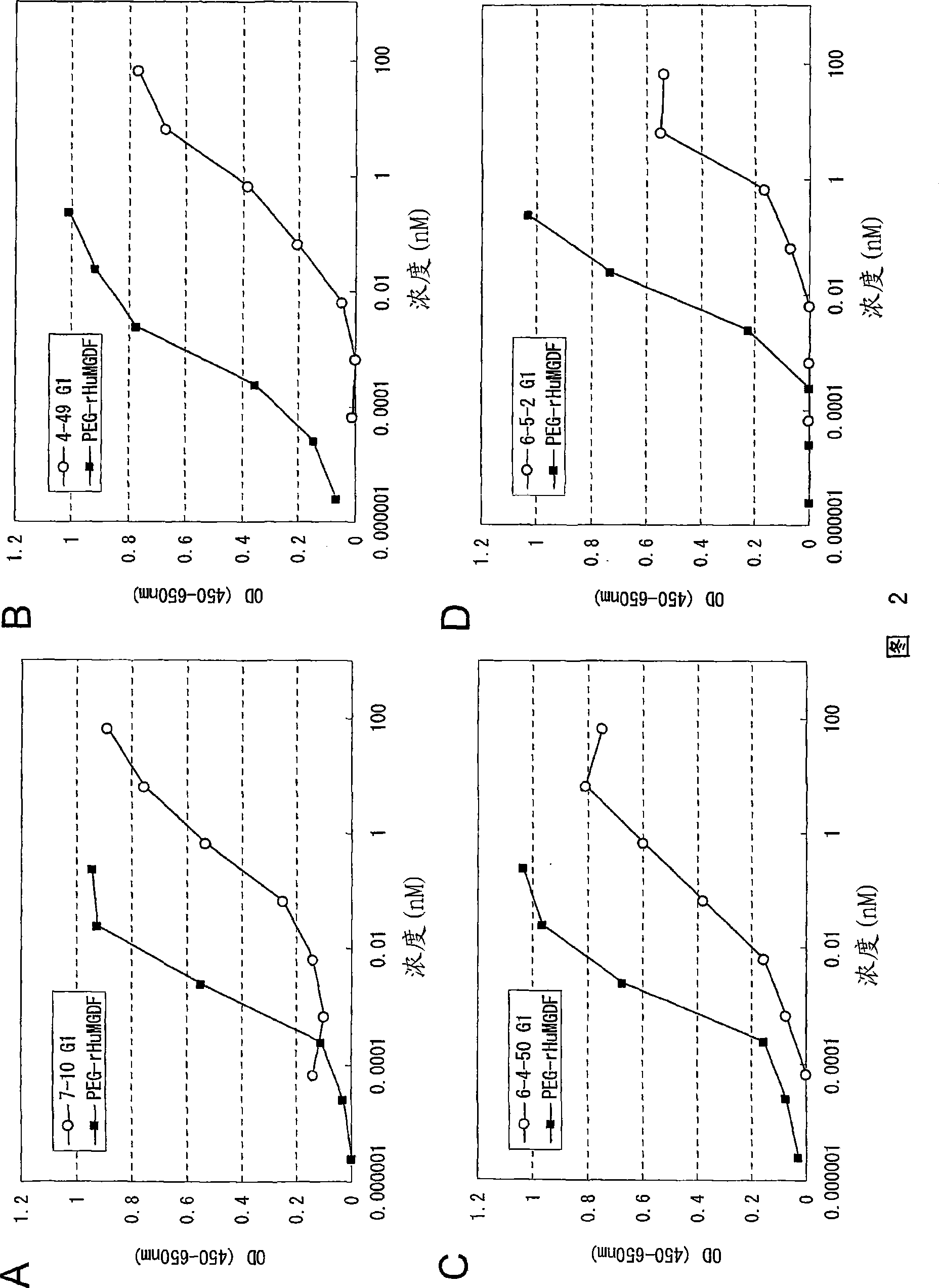
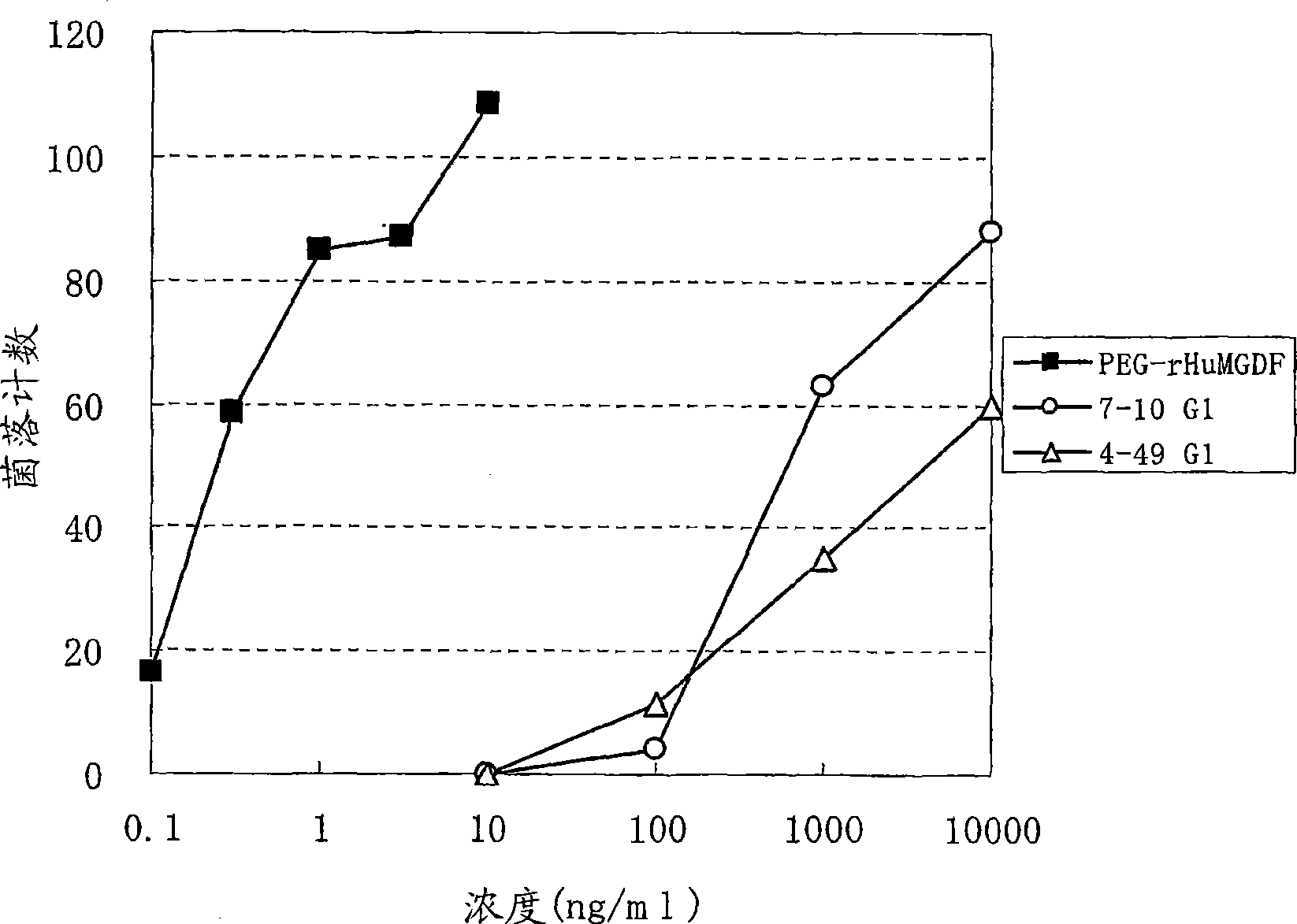
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Monoclonal antibody with function of blocking human programmed death factors 1 (PD-1), encoding gene of monoclonal antibody and application thereof
ActiveCN105566496AHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntiviralsAbnormal tissue growthAntigen
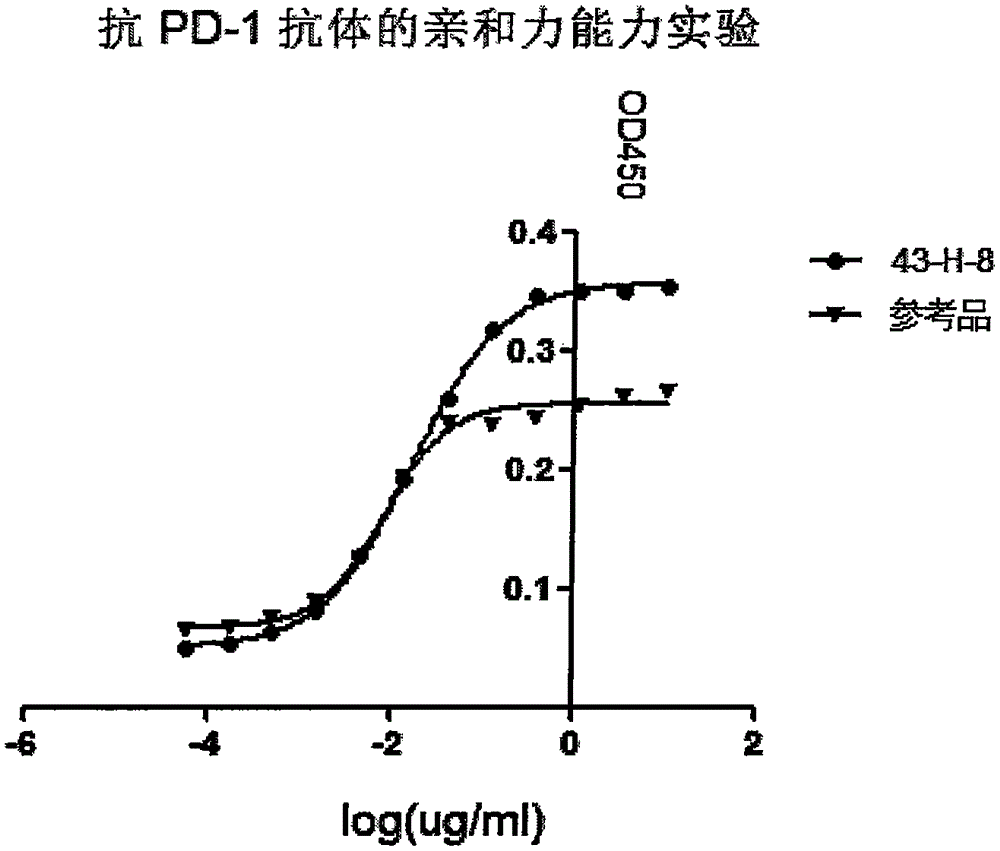
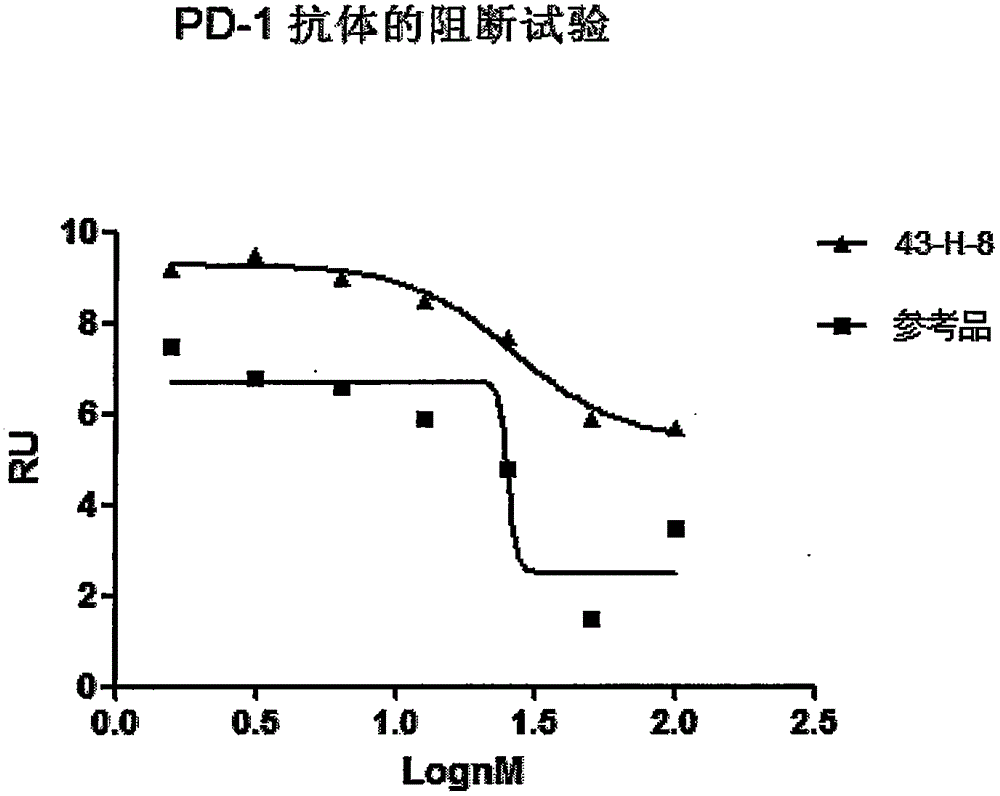
The invention discloses a monoclonal antibody with a function of blocking human programmed death factors 1 (PD-1), an encoding gene of the monoclonal antibody and application thereof. The monoclonal antibody comprises heavy chains and light chains. Amino acid sequences of the heavy chains are shown as 20 bits to 132 bits of SEQ ID NO.3, and amino acid sequences of the light chains are shown as 20 bits to 130 bits of SEQ ID NO.4. The monoclonal antibody 43-H-8 with the function of blocking the human programmed death factors PD-1, the encoding gene of the monoclonal antibody and the application have the advantages that the monoclonal antibody 43-H-8 can be specifically bound with human PD-1 antigens, the medium effective concentration EC50 (50% effective concentration) is 1.0665nM, accordingly, PD-1 / PD-L suppression signals can be specifically blocked, the monoclonal antibody 43-H-8 can be used as a blocker for PD-1 passages, and a novel medicine can be provided for tumor immunotherapy and treatment on chronic virus infectious diseases and autoimmune diseases.
Owner:大庆东竺明生物技术有限公司
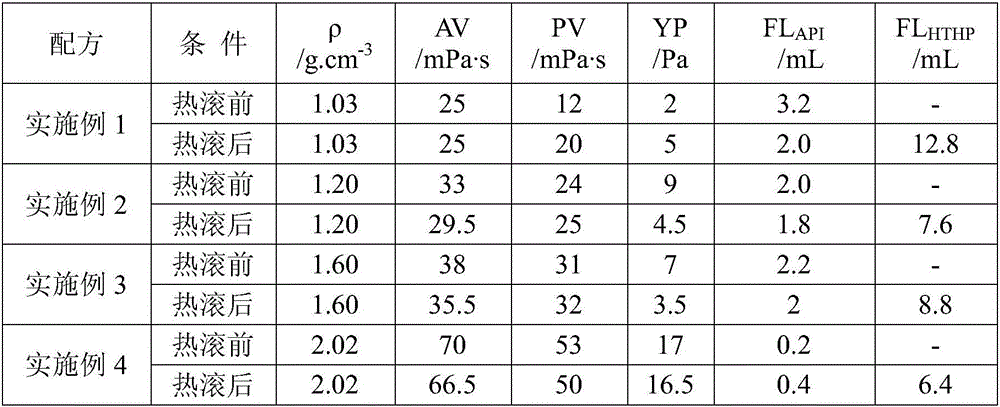
Environment-friendly water-based drilling fluid and preparation method thereof
ActiveCN106675535AImprove rheologyGood filter loss reduction effectDrilling compositionWater basedFiltration
The invention provides environment-friendly water-based drilling fluid and a preparation method thereof. The water-based drilling fluid is prepared from the following raw materials in parts by weight: 100 parts of water, 1-5 parts of bentonite, 0.05-0.4 part of sodium carbonate, 0.5-3 parts of a filtrate reducer A, 0.2-3 parts of a filtrate reducer B, 0.1-1 part of a viscosity / yield boosting agent, 0.5-4 parts of an inhibitor, 1-5 parts of a lubricant, 2-6 parts of a micro / nano particle plugging agent and 0-200 parts of weighting materials. The preparation method of the drilling fluid comprises the steps of adding bentonite and sodium carbonate into water; sequentially adding the filtrate reducer A, the filtrate reducer B, the viscosity / yield boosting agent, the inhibitor, the lubricant and the micro / nano particle plugging agent; adjusting the pH value by using sodium carbonate; selectively adding the weighting materials until the density reaches the drilling requirements. The resisting temperature of the drilling fluid can reach 150 DEG C, EC50 is greater than 3.0*10<4>mg / L, and the drilling fluid is nontoxic and environmentally friendly and has good rheological property, filtration reducing property, rejection capability, lubricating property, pollution resistance and reservoir protection effect.
Owner:BC P INC CHINA NAT PETROLEUM CORP +3
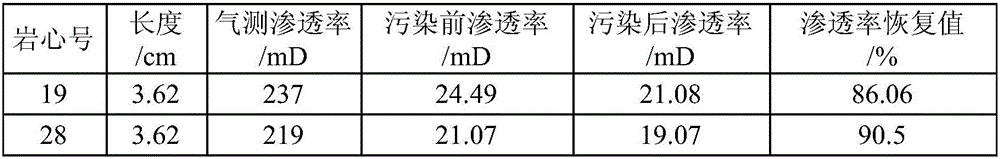
Seawater-based well completion fluid
The invention relates to seawater-based well completion fluid, which can effectively overcome the defects of the conventional well completion fluid in properties and application. A technical scheme for overcoming the defects is that: the seawater-based well completion fluid consists of clean seawater, a salt-resistant, thickening and fluid loss agent, an anti-collapse inhibitor, a well completionfluid lubricant, a reservoir protective agent, a well completion fluid preservative and a pH value regulator. The components of the seawater-based well completion fluid are environmentally-friendly additives, so that the seawater-based well completion fluid has the advantages of optimizing properties of well completion fluid and meeting the conventional properties (such as density, acidity and alkalinity, fluid loss property, rheological property, stability and the like), along with high biodegradability, no biological accumulation, low toxicity or no toxicity, environmental friendliness and the like. The EC50 value of the seawater-based well completion fluid is equal to 5.9*10<5> mg / L and the recovery value of core permeability reaches 88.7 percent, so that the aims of protecting the reservoir, protecting the environment and increasing the utilization ratio of petroleum and natural gas resources are fulfilled.
Owner:中石化石油工程技术服务有限公司 +1
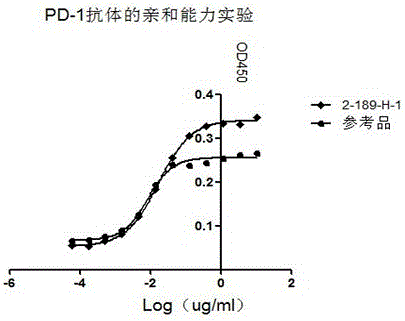
Preparation and application of anti-human programmed death factor 1 (PD-1) monoclonal antibody
ActiveCN106046162AHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenAutoimmune disease
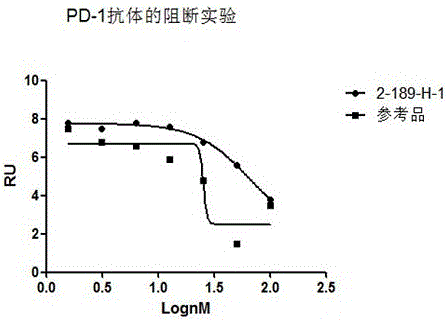
The invention discloses preparation, a variable-region sequence and application of an anti-human programmed death factor 1 (PD-1) monoclonal antibody. The invention provides the anti-human PD-1 monoclonal antibody which comprises gene sequences of heavy-chain variable regions of the monoclonal antibody and gene sequences of light-chain variable regions of the monoclonal antibody. The amino acid sequences of the heavy-chain variable regions are as shown in site 20th to site 134th of SEQ ID NO.3; the amino acid sequences of the light-chain variable regions are shown in site 20th to site 131th of SEQ ID NO.4. The invention discloses the monoclonal antibody 189-H-1 capable of blocking a human PD-1 function and a coding gene thereof. The monoclonal antibody 189-H-1 can be specifically combined with human PD-1 antigen, has median effective concentration EC50 being 1.0667 nM, can specifically block a PD-1 / PD-L inhibition signal, and therefore, the monoclonal antibody 189-H-1 can be used as a blocker of a PD-1 access, so that the anti-human PD-1 monoclonal antibody becomes a novel drug for tumor immune therapy, chronic virus infective disease treatment and autoimmune disease treatment.
Owner:大庆东竺明生物技术有限公司
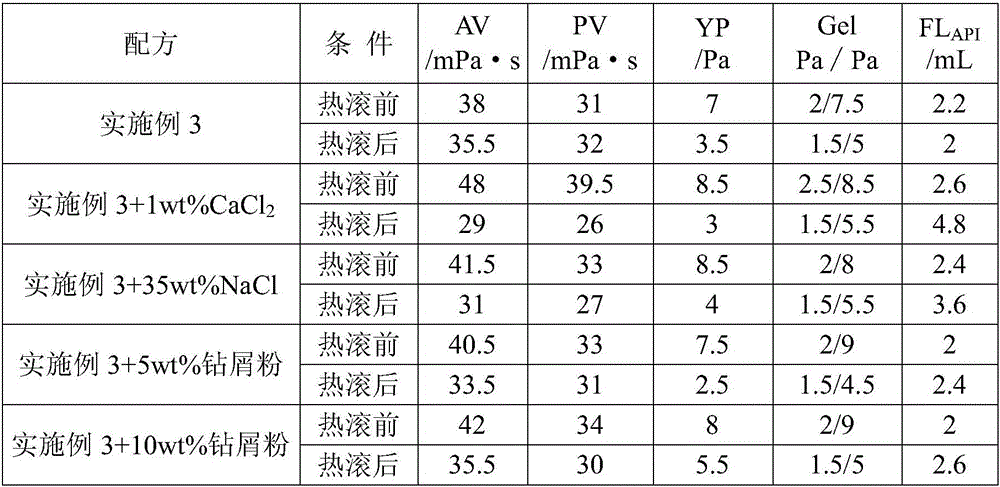
Environment-friendly, low-damage and clay-free deep water constant-rheology drilling fluid
The invention discloses an environment-friendly, low-damage and clay-free deep water constant-rheology drilling fluid comprising the following components in percentage by mass: 0.35-0.5% of alkalinity regulator, 0.4-0.5% of tackifying agent, 7-10% of inhibitor, 2.1-2.5% of filtrate reducer, 3-5% of lubricating agent, 0.3-0.5% of mud cake quality modifying agent, 0-0.8% of viscosity reducer, 0-61.2% of weighting agent and the balance of seawater. The environment-friendly, low-damage and clay-free deep water constant-rheology drilling fluid is free of biotoxicity, easy to biologically degrade and environment friendly; the EC50 value of the drilling fluid is larger than 120000mg / L, the rock core permeability recovery rate is 87.37%, a polluted segment is shorter than 0.5cm, the damage of foreign fluid to a hydrocarbon reservoir can be reduced, and the ocean environment can be effectively protected; the drilling fluid disclosed by the invention can resist to the temperature of 120 DEG C, has the controllable density of 2.0g / cm<3> and constant dynamic shearing force at different test temperatures after being subjected to hot rolling for 16h, and is favorable in thixotropy, relatively strong in shearing dilutability, easy to form a flat plate flow and strong in rock carrying capacity. By using the environment-friendly, low-damage and clay-free deep water constant-rheology drilling fluid, the in-well pressure kick, the ECD (Equivalent Circulating Density) value and the borehole slurry leakage can be effectively reduced, the slurry loss of a vibrating screen is avoided, the borehole cleanness is improved, and the non-production operation time is saved.
Owner:浙江仁智股份有限公司
Crack plugging agent based on nano-micron fibers for drilling fluid and preparation method
ActiveCN109868121AEffective blockingLittle impact on performanceDrilling compositionFiberAcute toxicity testing
The invention discloses a crack plugging agent based on nano-micron fibers for drilling fluid and a preparation method. The crack plugging agent comprises the following components in parts by mass: 32-37 parts of nano fibers, 6-7 parts of a dispersing agent and 57-61 parts of micron fibers. The preparation method mainly comprises the steps of bagasse pretreatment, micron fiber preparation, nano fiber preparation and preparation of the crack plugging agent based on nano-micron fibers. According to the invention, sugarcane residues are dried, crushed, purified, strongly acidified and dialyzed, and are combined with micron fibers on the basis to form a composite fiber plugging type, so that nano pores and micro cracks in shale can be effectively plugged. The plugging agent has little influence on the performance of the drilling fluid, is non-toxic, has no adverse effect on the environment, has the acute toxicity EC50 of more than 60000, and is an efficient shale micro-crack plugging agent.
Owner:CHINA PETROCHEMICAL CORP +3
Method for predicating embryotoxicity of non-steroidal anti-inflammatory drug type novel pollutants on early-phase life stage of zebra fish
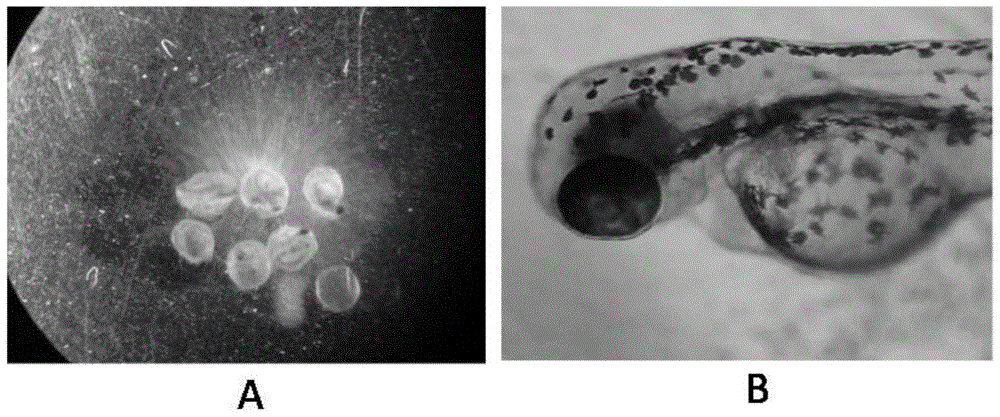
ActiveCN105044317AAddressing non-reflective typical NSAID sewage biotoxicityBiological testingLife stageFish embryo
The invention discloses a method for predicating the embryotoxicity of non-steroidal anti-inflammatory drug type novel pollutants on an early-phase life stage of zebra fish. The method comprises the following steps: exposing zebra fish embryos in the non-steroidal anti-inflammatory drug type novel pollutants which have equal logarithm space concentrations; recording the death rate and the aberration rate of the zebra fish embryos 7 days after exposing; and calculating by using SPSS software to obtain corresponding LC50 and teratogenetic EC50, which are used for evaluating the toxicity of the non-steroidal anti-inflammatory drug type novel pollutants. With the adoption of the method, the toxicity feature and the toxicity level of the non-steroidal anti-inflammatory drug type novel pollutants are subjected to an analytical test and quantitative description; and meanwhile, the method also can be used as an index for monitoring and evaluating the wastewater biological toxicity of non-steroidal anti-inflammatory drugs, and reference is provided for risk predication and evaluation of the potential biological toxicity of the pollutants in a water body.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY
Ecological risk evaluation method of PPCPs (pharmaceutical and personal care products) in water environment
InactiveCN106841431ALow costEasy to operateComponent separationPredicted no-effect concentrationTest organism
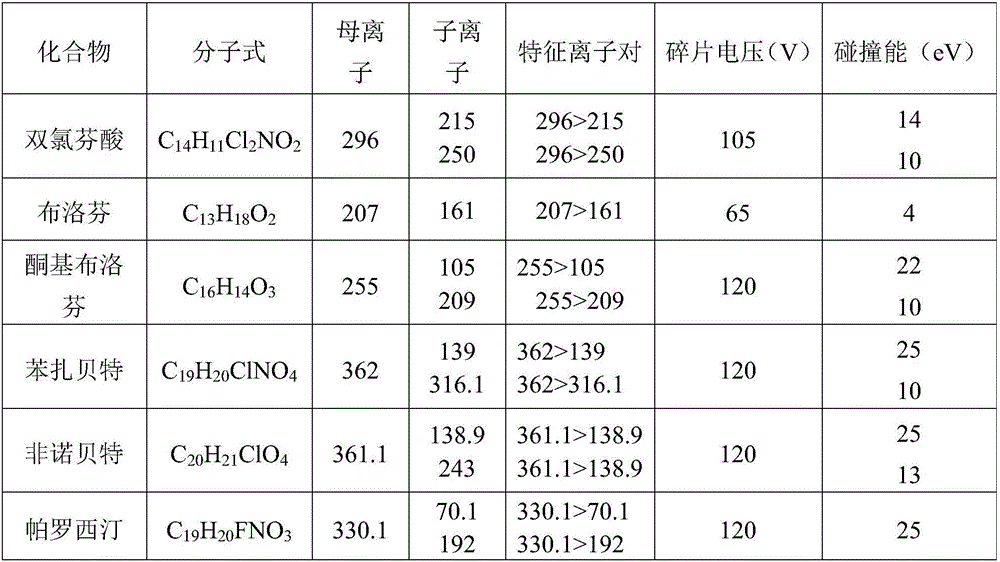
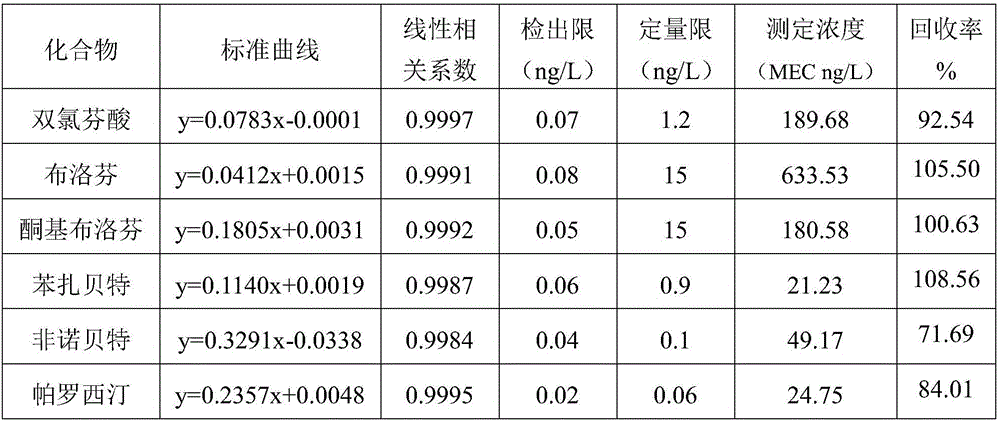
The invention discloses an ecological risk evaluation method of PPCPs (pharmaceutical and personal care products) in a water environment. The method comprises the following steps: (1) detection of PPCPs concentration in a water environment: water sample pretreatment, solid-phase extraction and quantitative detection by a high performance liquid chromatography-mass spectroscopy combined technique; (2) by using bacteria, algae, invertebrates, fish and other aquatic organisms as test organisms, determining the predicted no effect concentration (PNEC) by using toxicological data 50% effective concentration (EC50); (3) calculating the risk quotients (RQ) according to the formula RQ=MEC / PNEC, wherein MEC is the measured environmental concentration; and (4) evaluating the ecological risk degree of the target compounds PPCPs by an RQ process. The method is low in cost, friendly to the environment and convenient for operation. The detection method of PPCPs is simple to operate and easy to implement, has the advantages of high selectivity for target compounds, high sensitivity, high enrichment multiple and favorable reproducibility, and can simultaneously separate and determine multiple compounds.
Owner:TIANJIN UNIV
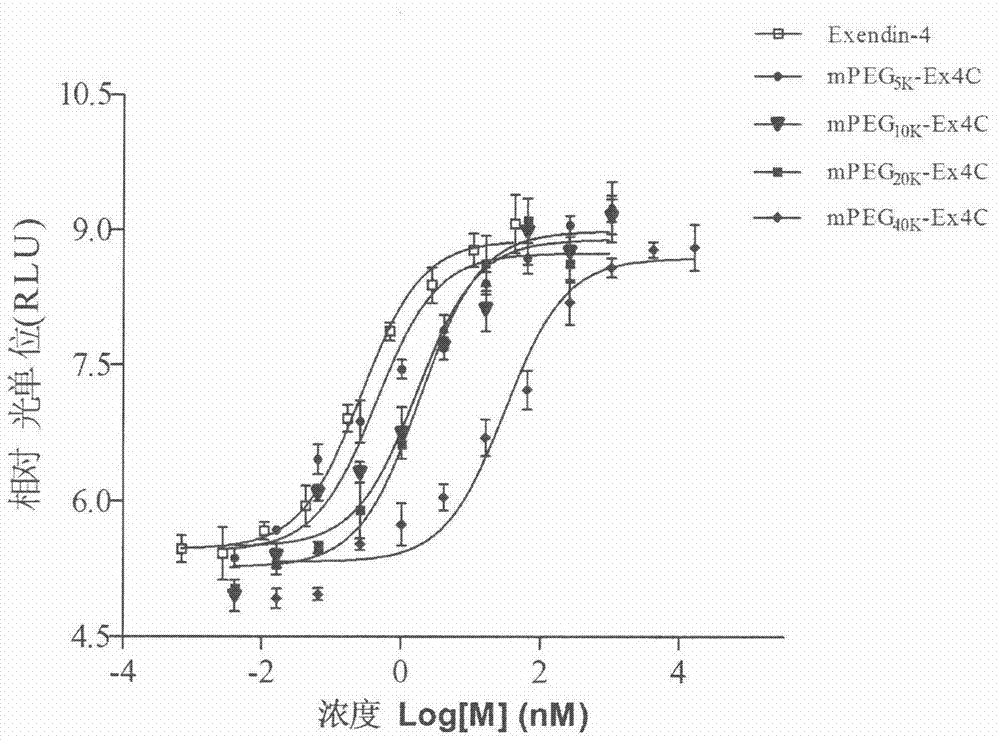
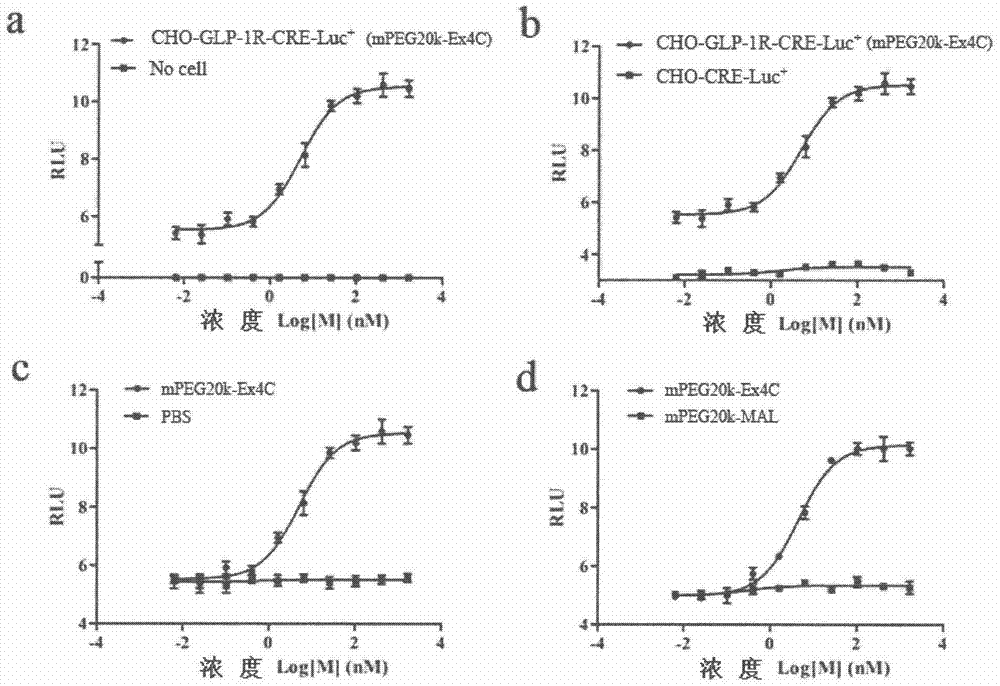
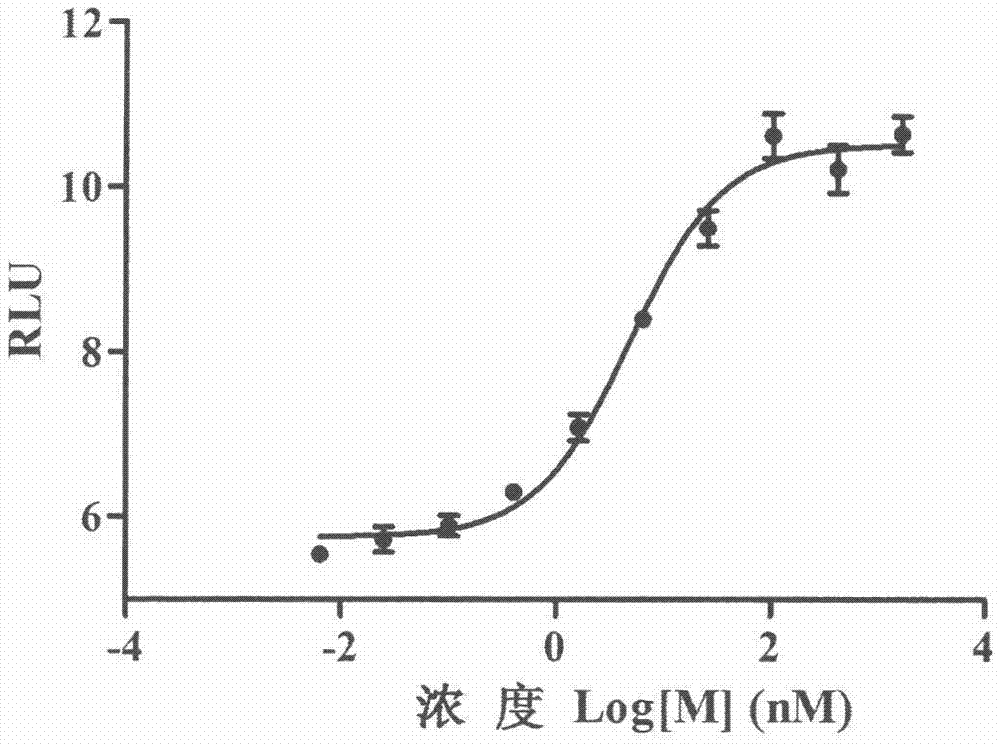
Method for determining receptor affinity of GLP-1 receptor agonist
InactiveCN104846061AQuick evaluationQuick filterMicrobiological testing/measurementCytosolResponse element
The invention provides a method for determining the receptor affinity of a GLP-1 receptor agonist. The method is based on a tool cell line, namely CHO-GLP-1R-CRE-Luc<+>, when a sample to be determined is combined with GLP-1 receptor on the surface of a cell, the receptor-mediated signal cascade reaction can be activated, cAMP-response element (CRE) is activated specifically, the expression of the luciferase reporter gene is promoted, the quantity of luciferase in cytosol is detected for drawing and fitting to obtain a dose-effect curve of acting of the sample and the GLP-1 receptor; and the half effective concentration (EC50) is calculated. By inspecting and optimizing all influence factors in the determining process, the method for determining the receptor affinity of the GLP-1 receptor agonist is finally established. The method has the advantages of being high in specificity, precision and accuracy, good in durability and convenient to operate, and the like. The method can be used for determining the receptor affinity of the GLP-1 receptor agonist, and thus such type of drug can be fast evaluated and screened.
Owner:CHINA PHARM UNIV
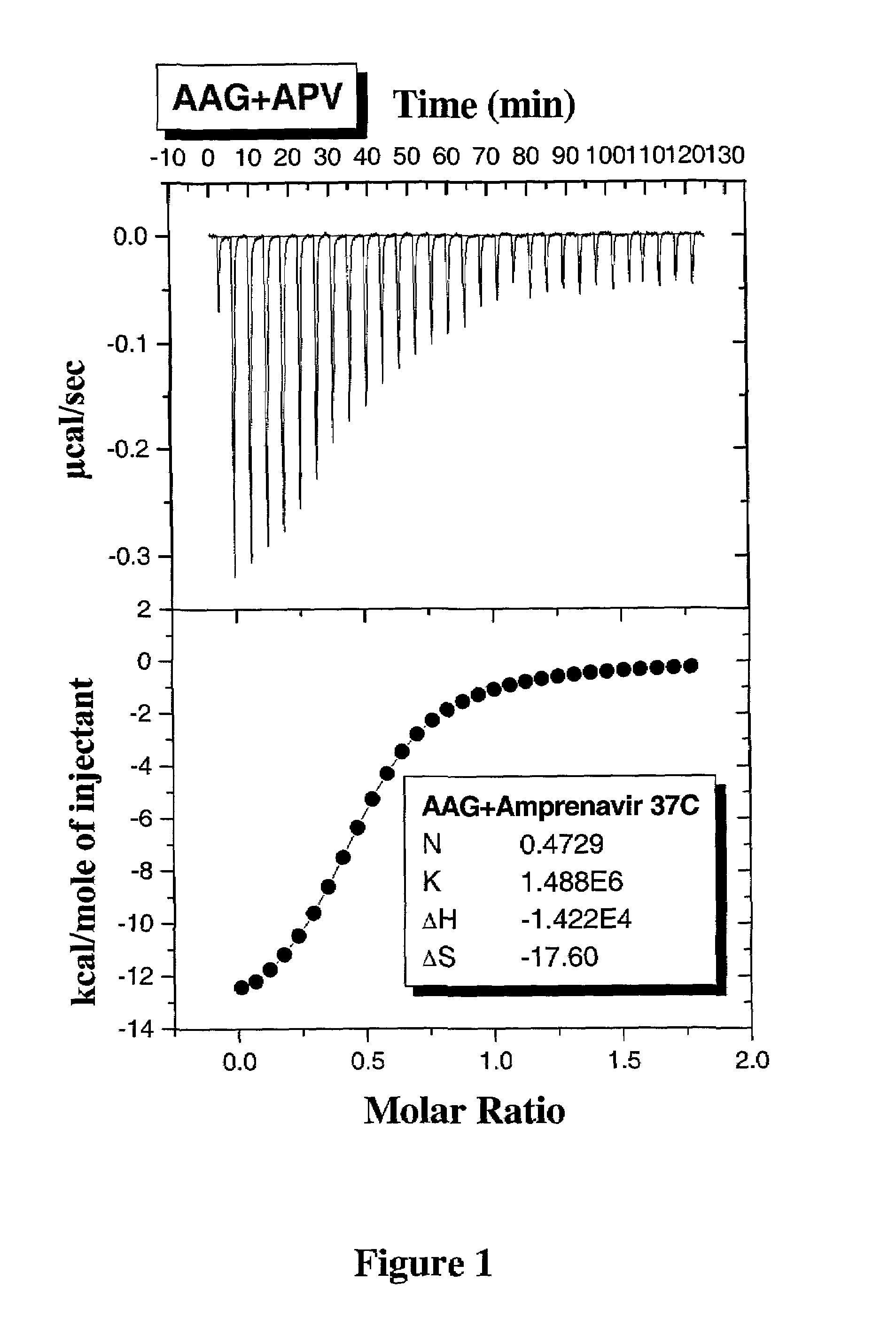
Methods for determining plasma free drug concentration by direct measurement of binding affinity of protease inhibitors to plasma proteins
InactiveUS7087373B2Evaluate the in vivo anti-HIV efficacy of PIsCompound screeningApoptosis detectionRegimenIn vivo
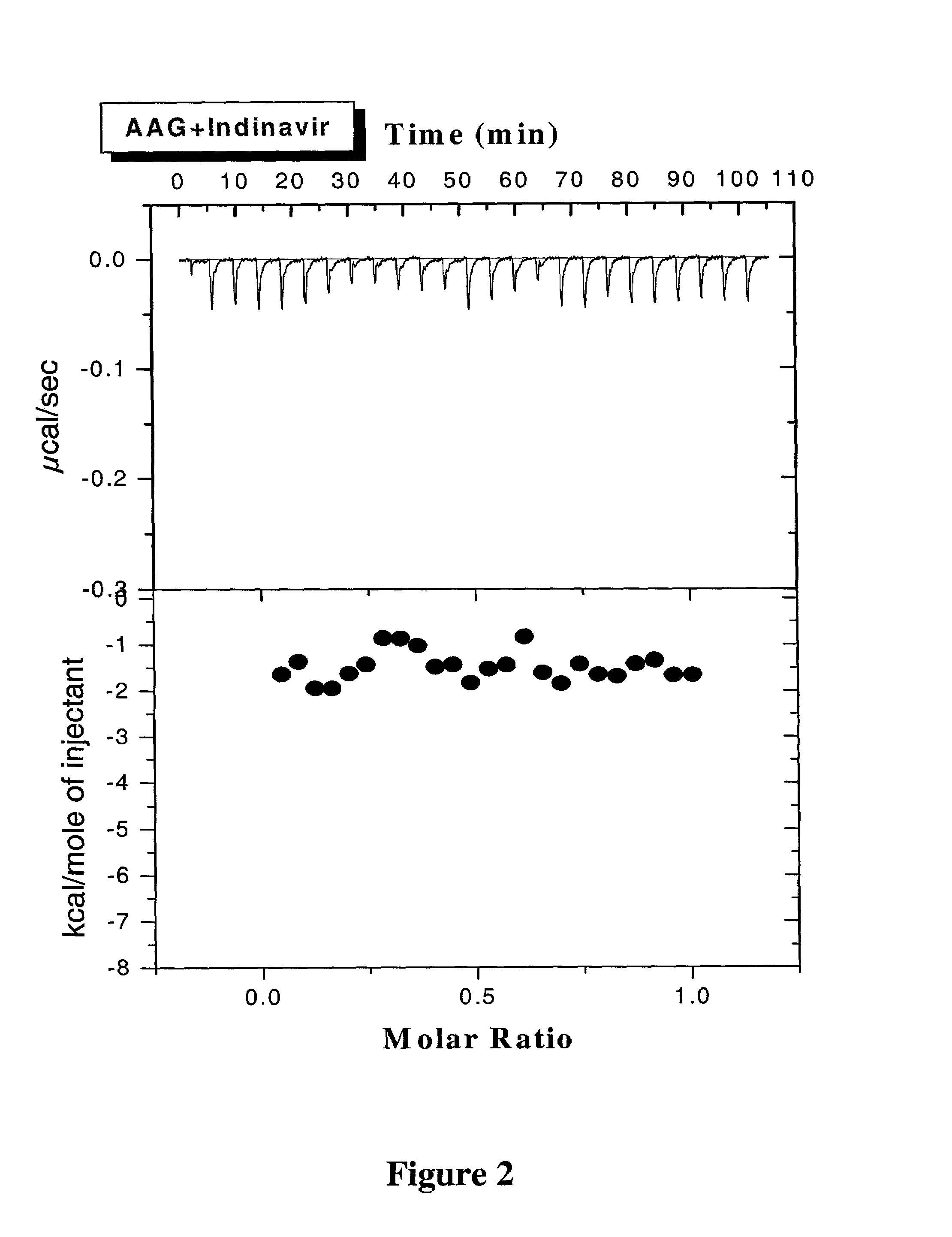
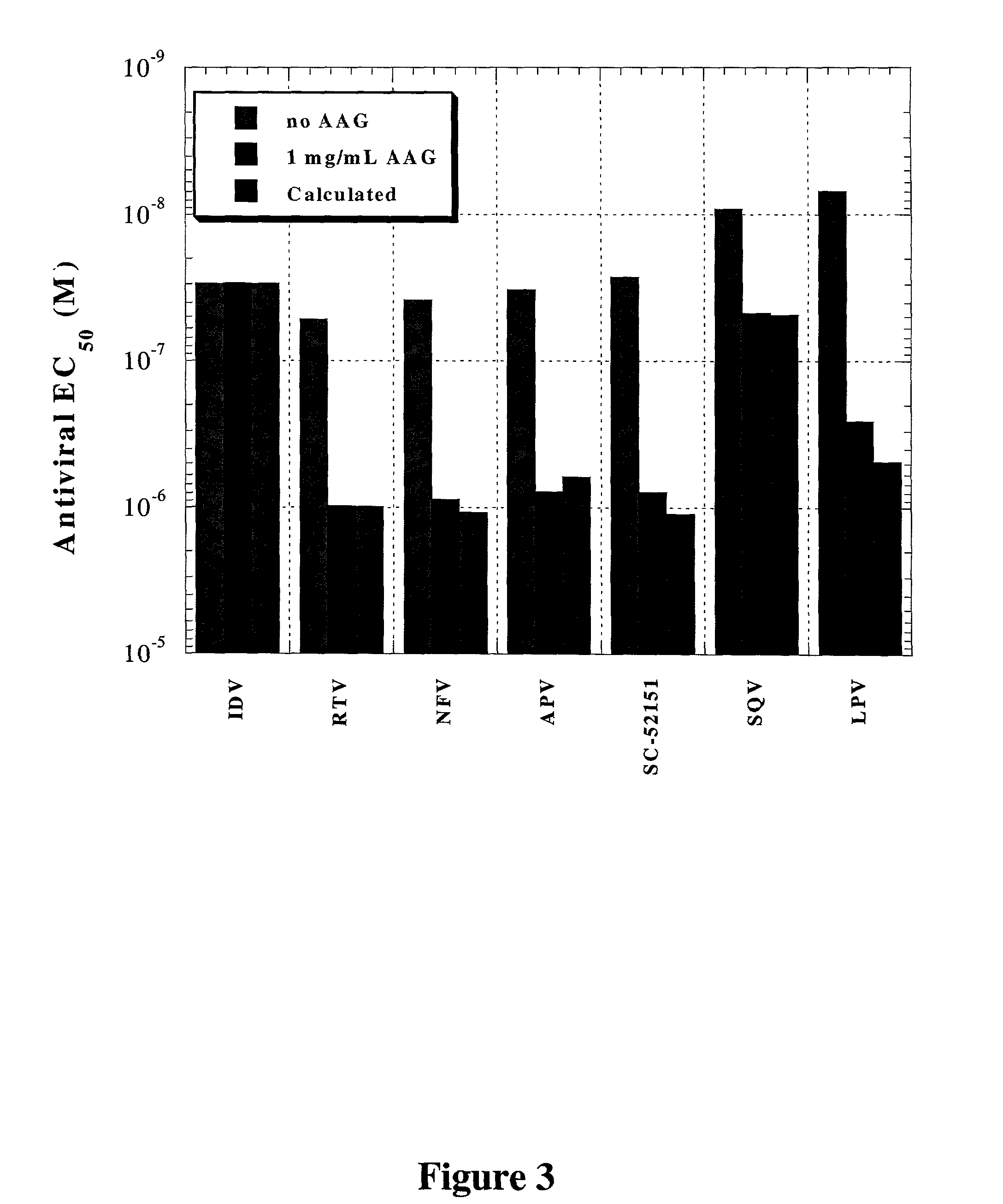
Methods for isothermal titration calorimetry analysis of the binding affinity of protease inhibitors to plasma proteins. A method that can quantitatively calculate free drug concentrations of protease inhibitors in human plasma, as well as a method to calculate therapeutic amounts and dosage regimens. Furthermore, the present invention provides a method that can calculate the effect of plasma proteins on the antiviral activity (EC50 values) of protease inhibitors from their binding affinities to plasma proteins. The present invention provides as well a method that can evaluate the in vivo anti-HIV efficacy of PIs in human plasma.
Owner:TIBOTEC PHARMA
Application of alpha-mangostin in controlling plant diseases
ActiveCN103999859ABroad-spectrum bactericidal activityGood antibacterial effectBiocideFungicidesSpore germinationTherapeutic effect
The invention discloses an application of alpha-mangostin in controlling plant diseases. Tests prove that alpha-mangostin has agricultural fungicidal activity. The compound has very strong fungistatic action on mango anthracnose pathogens, diplodina sp., botrytis cinerea, and exserohilum turcicum when the testing concentration is 100 mg / L, and has an EC50 value of 18 mg / L-35 mg / L; the compound has certain spore germination inhibition effect on exserohilum turcicum and botrytis cinerea, and the EC50 values are 66.20 mg / L and 50.43 mg / L respectively; meanwhile, in-vivo test results show that the compound has very good protection and treatment effects on rubber tree powdery mildew when the testing concentration is 500 mg / L, and the protection and treatment effects on rubber tree powdery mildew are 75.61% and 70.12% respectively, which are higher than those of a control drug of prochloraz. The above results indicate that alpha-mangostin has broad-spectrum fungistatic action on plant pathogenic fungi, which lays the foundation for the development of novel pollution-free plant-derived pesticides by further using mangosteen shells.
Owner:ENVIRONMENT & PLANT PROTECTION INST CHINESE ACADEMY OF TROPICAL AGRI SCI
Method for preparing water soluble chitosan oligosaccharide from squid sheath and bioactivity of chitosan oligosaccharide
InactiveCN102875694AIncrease concentrationEasy to useSugar derivativesDigestive systemDPPHWater soluble chitosan
The invention discloses a method for preparing water soluble chitosan oligosaccharide from squid sheath. The method is characterized by comprising the following steps of: crushing the squid sheath, adding 6 to 9 percent HCl solution, ensuring that a material / solution (w / v) ratio is 1:14, decalcifying for 20 to 60 minutes, washing to neutrality, adding 10 percent NaOH solution, ensuring that a material / solution (w / v) ratio is 1:10, removing protein, filtering and washing to neutrality, and drying to obtain beta-chitin; adding 30 to 50 percent NaOH solution into the beta-chitin, ensuring that a material / solution ratio (w / v) is 1:10, performing ultrasonic bath reaction at the temperature of between 70 and 100DEG C for 2 to 4 hours, filtering, washing to neutrality, and drying to obtain chitosan; and adding 3 to 12 percent H2O2 into the chitosan, ensuring that a material / solution ratio (m / v) is 1:15-1:25, reacting at the temperature of between 45 and 75DEG C for 2 to 5 hours in the assistance of ultrasonic waves, concentrating filtrate, adding ethanol in an amount which is 3 to 5 times volume of the concentrated filtrate, precipitating and filtering a product, and drying to obtain the chitosan oligosaccharide, wherein the chitosan oligosaccharide has the molecular weight of less than 1*10<4>Da, the DPPH free radical removing capacity (EC50) of between 0.3 and 1.3mg / mL and the cholic acid combining capacity of between 20.47 and 63.53mg / g. The invention provides technical support for comprehensively utilizing leftovers, namely the squid sheath in aquatic product processing and industrially producing the water soluble chitosan oligosaccharide.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY
Screening and application method of small-molecule inhibitor capable of inhibiting proliferation of toxoplasma gondii
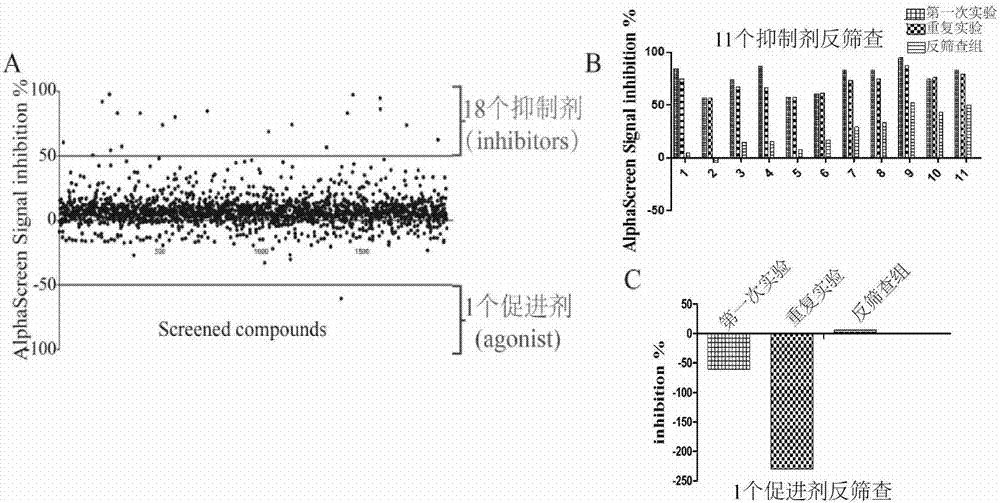
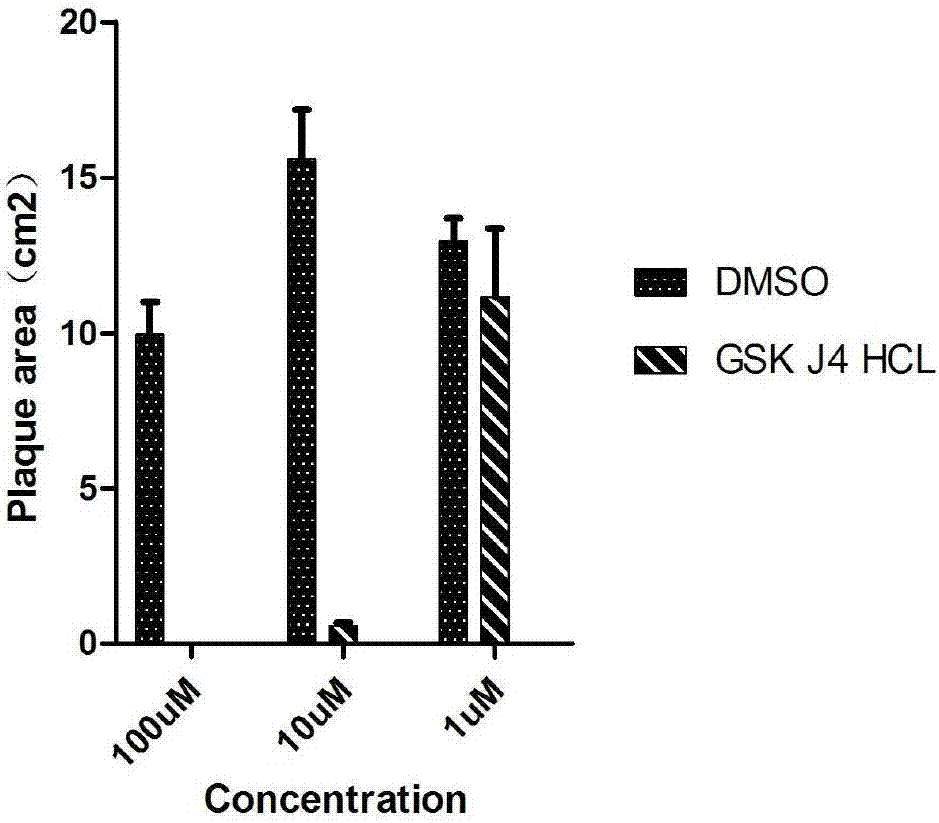
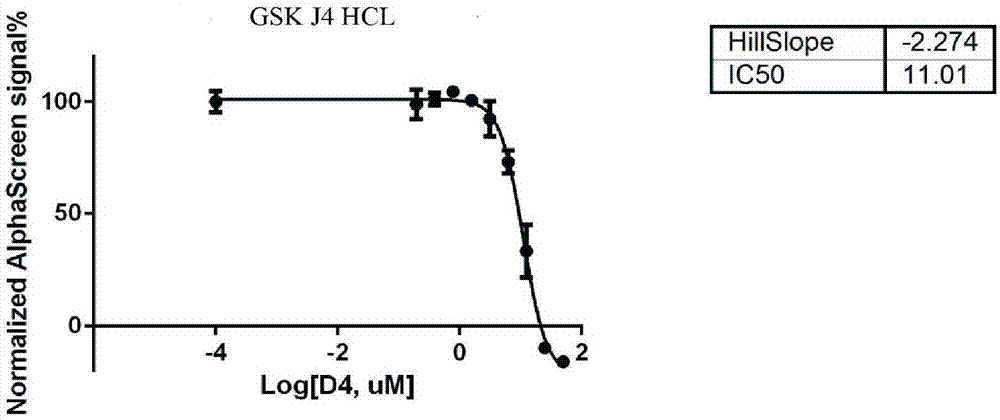
InactiveCN107884587APrevent proliferationR&D cost economyBiological testingScreening methodAccelerant
The invention discloses a screening method of small-molecule inhibitor capable of inhibiting proliferation of toxoplasma gondii. The method utilizes an Alphascreen experiment to primarily select 11 small-molecule inhibitors and one small-molecule accelerant, and the 12 drugs are used for clinical treatment. The 12 drugs are further screened at the cellular level, a plaque experiment proves that GSK J4HCL apparently inhibits the proliferation of toxoplasma gondii, and the value EC50 of the GSK J4HCL to toxoplasmagondii is determined to be about 4.5 muM by virtue of intracellular proliferation experiment. The AlphaScreen competitive inhibition experiment evaluates that the value IC50 of the GSK J4HCL for inhibiting the interaction of TgAtg8 to TgAtg3 is 11.01 muM. The CCK8 experiment is usedfor proving that the value IC50 of the drug for inhibiting the growth of a host cell is 34.359 muM which is far greater than the value EC50 of the drug for the insect, which illustrates that the GSKJ4HCL is a low-toxicity and high-efficiency drug for resisting the toxoplasma gondii.
Owner:WENZHOU MEDICAL UNIV
Method for detecting toxicity of characteristic toxic and harmful pollutants of aquatic products
InactiveCN102121935ADetection of absolute toxicityTesting waterTesting foodLuminous intensityRegression analysis
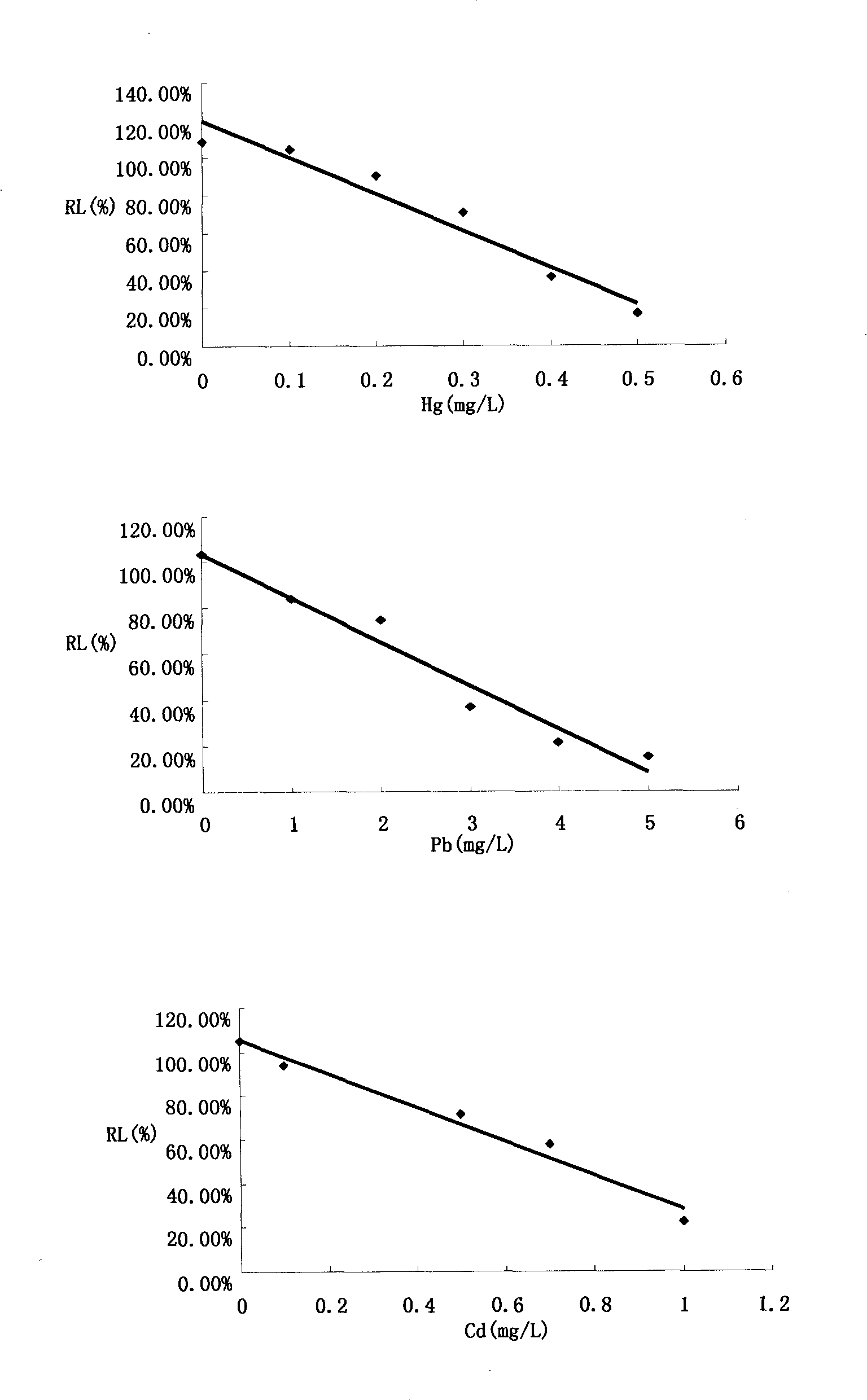
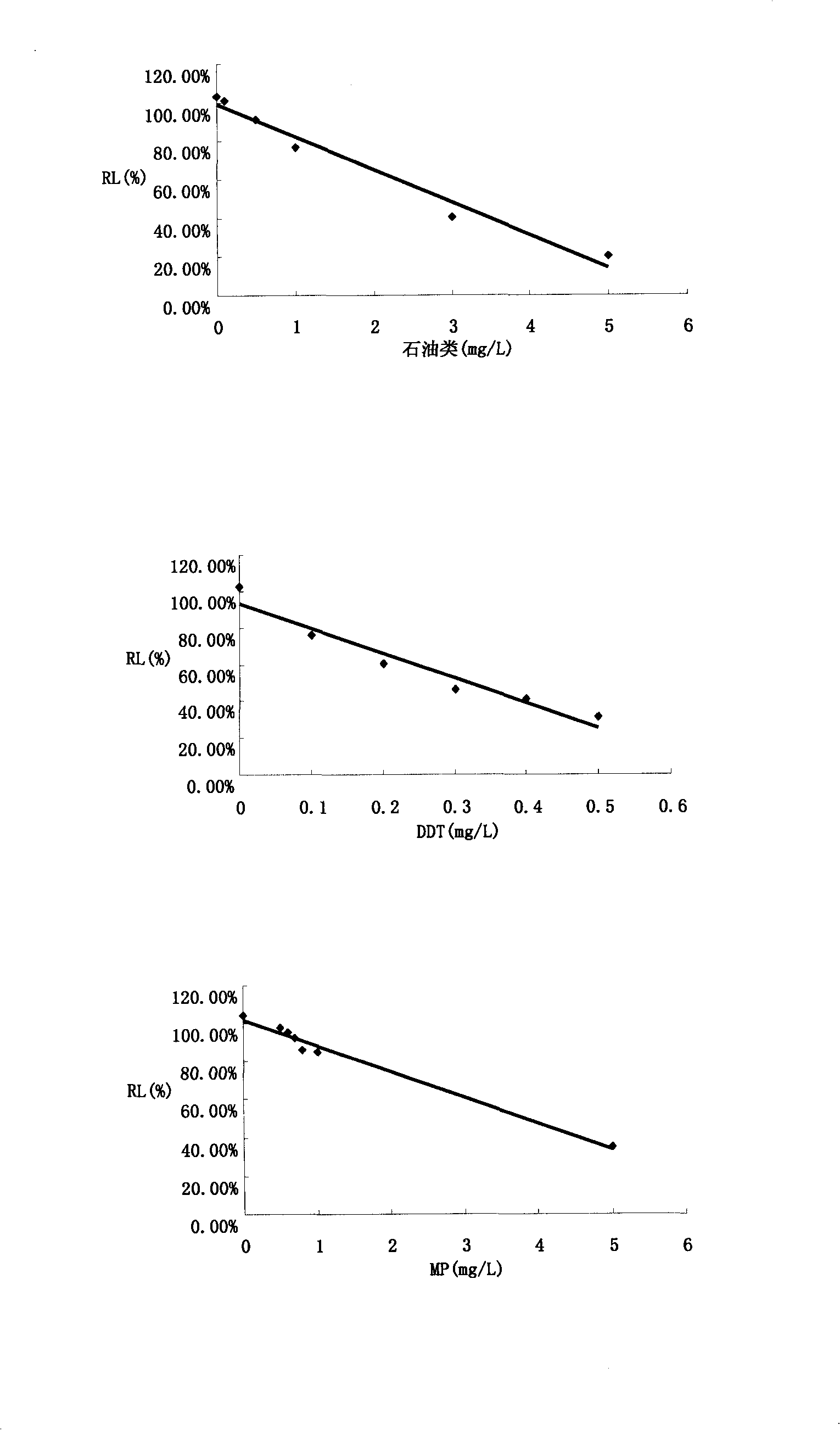
The invention relates to a method for detecting the toxicity of characteristic toxic and harmful pollutants of aquatic products. The method is characterized by comprising the following steps of: (1) measuring the relative luminous intensity (RL) of luminous bacteria in a polluted environment of toxic substances with different concentrations by adopting a half lethal concentration method in a toxicity detection analyzer; (2) solving a linear regression equation, i.e. Y=aX+6, through regression analysis by taking the concentrations of various toxic substances in the polluted environment as a horizontal coordinate and the RL as a vertical coordinate, solving the diluted concentration, i.e. an EC50 value, of a corresponding pollutant when the RL is 50%, and defining the EC50 value as a relative toxicity factor (RT); (3) testing the BCF (Biological Concentration Factor) of the various toxic substances in the polluted environment; and (4) carrying out division on the obtained RT and the BCF, wherein an obtained value is the absolute toxicity magnitude of the various toxic substances in the polluted environment. The detection method provided by the invention can be used for accurately and fast sequencing the toxicity of various pollutants on the aquatic products.
Owner:NINGBO UNIV
Cereal fermented nutrition powder and production method thereof
InactiveCN106418164AComprehensive and balanced nutritionHigh in nutrientsFood ingredient functionsBiotechnologyDPPH
The invention provides cereal fermented nutrition powder and a production method thereof. The production method comprises the following steps: taking mixture of cereals (tartary buckwheat, common buckwheat, rice, millet, black rice and black beans) as a raw material; taking edible mushroom agaricus blazei murill, lucid ganoderma or coprinus comatus as a fermented strain; and performing a solid fermentation process for preparing a solid fermentation product containing edible mushroom mycelia and metabolites thereof. The nutritional ingredients of the acquired fermentation product and the content thereof are as follows: 16.05%-17.64% of protein, 2.98%-3.84% of fat, 3.51mg / g-5.86mg / g total phenols and 20mg / g-91.50mg / g reducing sugar. According to the invention, on the basis of basically maintaining the original nutrition of cereals, the active ingredients of the edible mushroom mycelia are added, so that the product has reasonable nutritional composition and higher oxidation resistance. The EC50 value of the DPPH free radical scavenging capacity of the nutrition powder ethanol extract is 0.12-1.69, and the EC50 value of the Fe<2+> chelation capacity is 0.35-3. The acquired product tastes nice and has strong fragrance.
Owner:SHANXI UNIV
Plant-sourced bactericide for controlling bacterial wilt of tobacco
InactiveCN102388925AGood effectImprove the effect of disease preventionBiocideFungicidesNicotiana tabacumSterile water
The invention belongs to the technical field of agricultural chemicals, particularly relates to the field of plant-sourced bactericides and specifically provides a plant-sourced bactericide for controlling bacterial wilt of tobacco. The main active constituents of the plant-sourced bactericide for controlling bacterial wilt of tobacco are amaranth crude extract and sterile water, wherein each milliliter of the plant-sourced bactericide contains 0.17-0.5 mg of the amaranth crude extract. The plant-sourced bactericide for controlling bacterial wilt of tobacco has the advantages as follows: 1, the raw material of the amaranth crude extract is convenient to obtain and the amaranth crude extract can be produced industrially and can be applied by farmers; 2, the effect of the plant-sourced bactericide is obvious and the median inhibitory concentration EC50 is 0.0415 mg / mL; the effect of the plant-sourced bactericide for controlling bacterial wilt of potted tobacco is 77.00-90.29 percent andis obviously higher than the effects of streptomycin sulphate and other conventional plant-sourced bactericides for controlling bacterial wilt of tobacco; and 3, the amaranth crude extract is high inefficiency, low in toxicity, nuisanceless, and safe to environment.
Owner:HUNAN AGRICULTURAL UNIV
Algistat
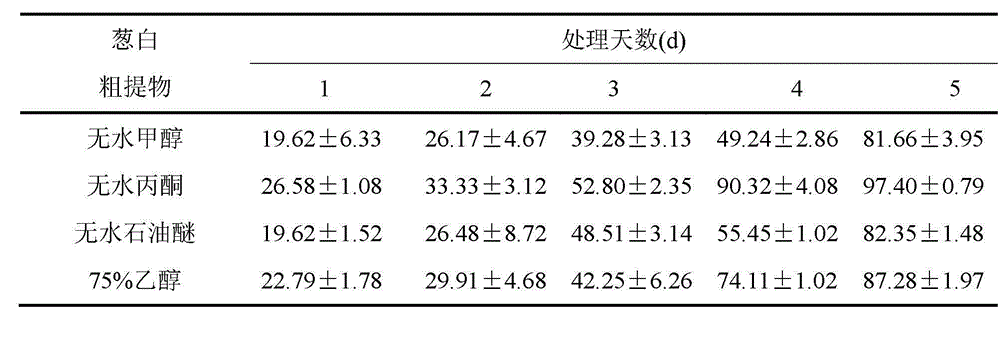
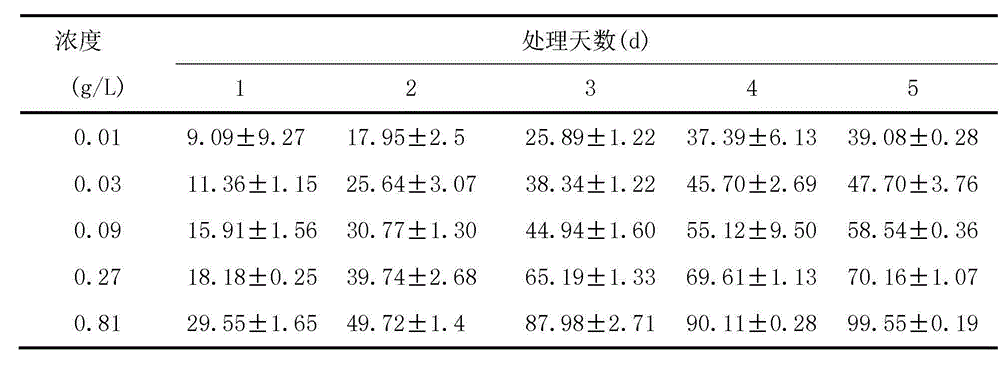
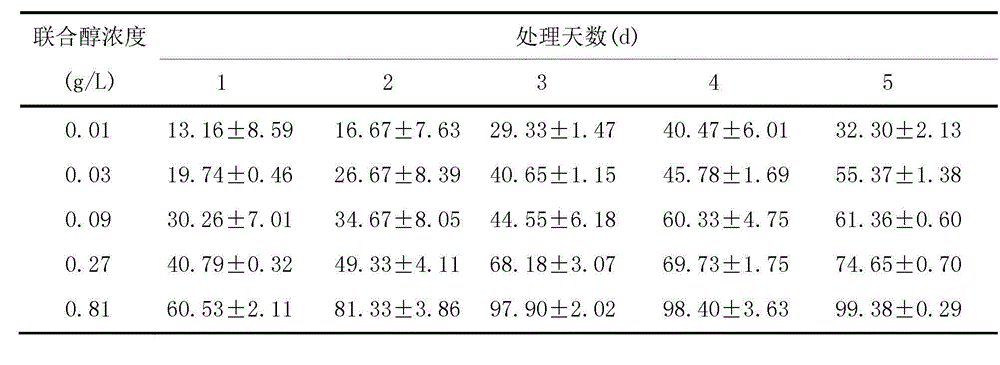
The invention discloses an algistat, which is an absolute methanol crude extract of onion stalks, an absolute acetone crude extract of the onion stalks, an absolute petroleum ether crude extract of the onion stalks or an ethanol crude extract of the onion stalks. Compared with the prior art, the crude extracts from the onion stalks by the organic solvents perform an inhibitory effect to microcystis aeruginosa from high to low as follows: the inhibitory effect of absolute acetone is larger than that of 75% ethanol, the inhibitory effect of the 75% ethanol is larger than that of absolute petroleum ether, and the inhibitory effect of the absolute petroleum ether is larger than that of absolute methanol. The inhibition ratio of the absolute acetone pure extract of the onion stalks is enhanced along with the time, and a concentration effect is shown. The inhibition ratio of a high dose group on the fourth day can be up to 97.7%. EC50 (50% effective concentration) computed on the fifth day is 0.03g / L, which proves that the algal inhibition effect of the purified absolute acetone extract is more obvious than that before purification.
Owner:ANHUI NORMAL UNIV
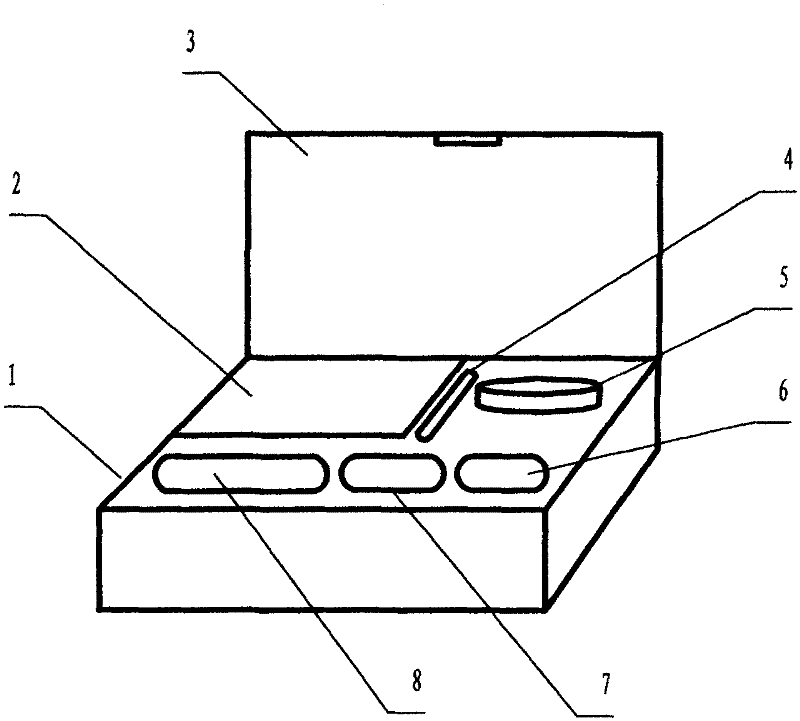
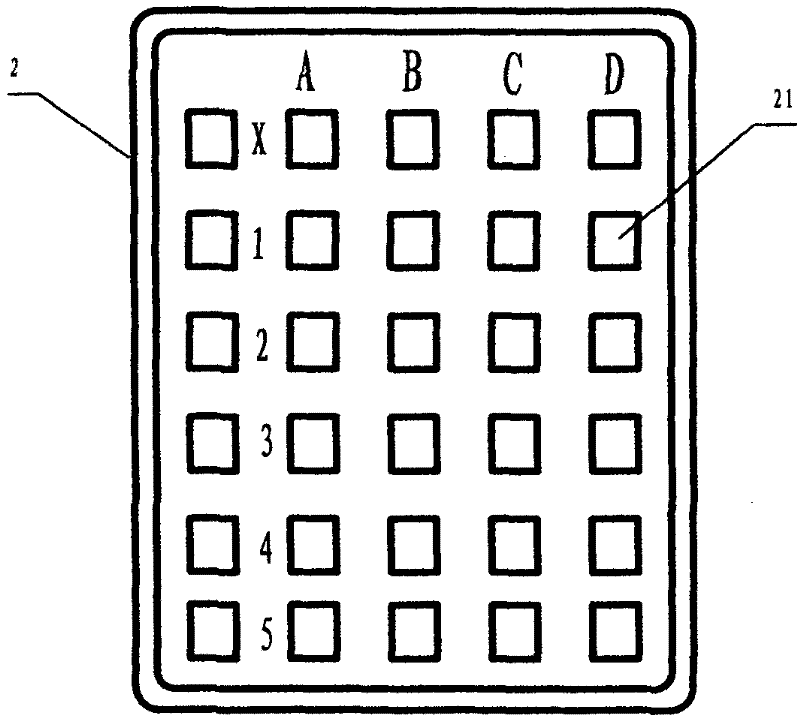
Kit for measuring acute toxicity of aqueous substances by using large siphonaptera, and manufacturing method and using method thereof
ActiveCN102183619AReduce detection errorSave labor costTesting waterAcute toxicity testingWater quality
The invention discloses a kit for measuring acute toxicity of aqueous substances by using large siphonaptera, and a manufacturing method and a using method thereof. The kit is provided with a shell, a test board, an incubation vessel, a dropper, a screen, a diluted jellyfish liquid bottle, a bait bottle and a large siphonaptera bottle. The manufacturing method of the kit comprises the following steps of: 1, preparing larvae of the pure strain large siphonaptera; 2, feeding the larvae; 3, separating adult siphonaptera; 4, preparing resting eggs; 5, purifying and storing the resting eggs; 6, preparing baits; 7, preparing diluted jellyfish liquid; and 8, manufacturing accessories and placing the products into boxes. The using method of the kit comprises the following steps of: 1, preparing standard diluted water; 2, incubating eggs of the large siphonaptera; 3, according to a result of a preliminary test, determining the concentration range of an official test of measuring the acute toxicity of the aqueous substances by using the large siphonaptera; 4, performing blank control; 5, starting to perform the test of measuring the acute toxicity of the aqueous substances by using the large siphonaptera; and 6, calculating the result, namely estimating semi-effect concentration EC50. The invention is suitable for measuring the acute toxicity of the aqueous substances by using the largesiphonaptera, low in cost, convenient in use and accurate in measurement.
Owner:BEIJING DRAINAGE GRP CO LTD
Screening method of high flux 96 orifice plate for herbicide
InactiveCN101126712AReduce usageSimple and fast operationMicrobiological testing/measurementColor/spectral properties measurementsContinuous lightScreening method
The utility model provides a screening method used for herbicide high flux 96-hole orifice plate. The utility model adopts the technical proposal that: firstly, exponential phase chlorella is inoculated on the 96-hole orifice with a culture medium which is suitable for the chlorella, and the initial inoculating number of the chlorella is 7 to 8 x 105 per mL; secondly, the concentration gradient herbicide is dipped into each hole, and each concentration is provided with two to five duplicate samples as well as an empty control sample. The culturing condition is that: temperature is 25 plus or minus 0.2 DEG C, light is 2000Lx, continuous light is kept, fresh-keeping film is used to seal, no nutrient solution is added, chlorella solution is aerated four to five times every day, the chlorella is cultivated 72 to 144 hours in total, test sample is measured at 630nm absorbance every day, and then the EC50 (median effect concentration) of the herbicide on the chlorella is calculated according to the relationship between the absorbance and the herbicide concentration. Compared with the flask method, the utility model has the advantages of reducing herbicide amount in screening, and having easy operation, fast speed and high sensitivity.
Owner:ZHEJIANG UNIV OF TECH
LeY SPECIFIC BIOTHERAPEUTIC
InactiveUS20120010388A1Weak affinityGood curative effectImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationEpitopeBinding site
Embodiments are related to the field of immunology, and provide a highly avid LeY specific biotherapeutic including at least one further binding site having a different specificity to bind an epitope of a glycosylated cell surface molecule of a tumor cell, characterized by EC50 of less than 1 mM to confer immediate cytotoxicity to the tumor cell.
Owner:HIMMLER GOTTFRIED
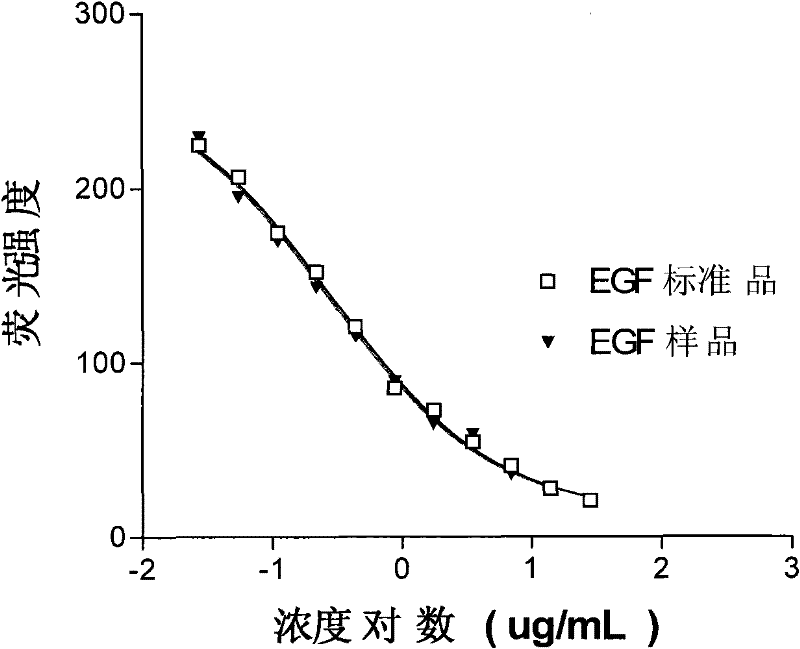
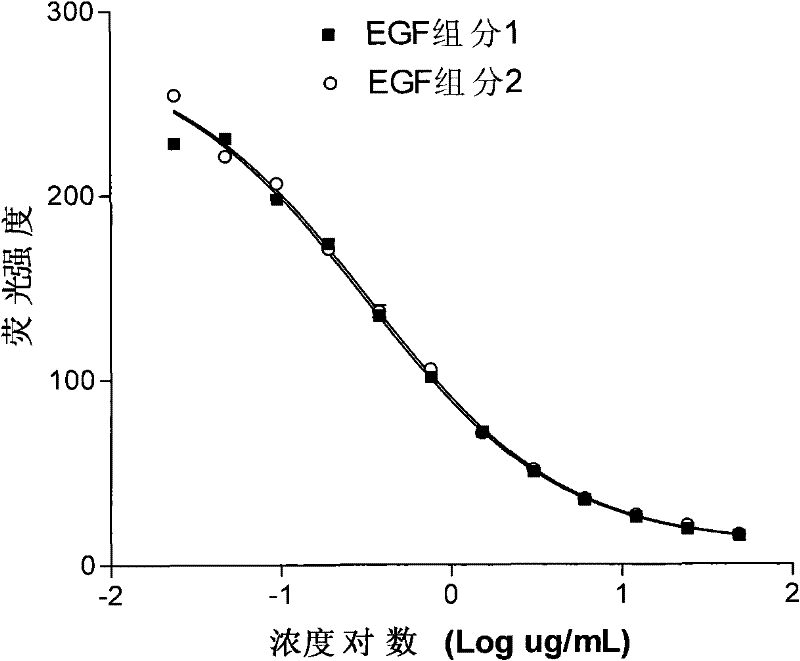
Determination method and application of biological activity of recombinant human epidermal growth factor
The invention discloses a method for measuring the biological activity of a recombined human epidermal growth factor (rhEGF) and application of the method. The method comprises the following steps of: blocking the combination between the EGF and the EGFR by an antibody to indirectly measure the biological activity of EGF and EGF receptor (EGFR) combination by a competitiveness-restrained flow cytometry; processing experimental data by adopting a computer program (four-parameter regression) to acquire half effective inhibition concentration (EC50) of an EGF standard substance and an EGF samplerespectively and thus obtaining the relative biological activity of the EGF sample; and calculating the biological activity of the EGF sample according to the biological activity (IU / mL) of the EGF standard substance. In the method, the test period is only one day from sample injection except cell pre-culturing; a germfree environment is not needed during test; and compared with the biological activity measurement method for the rhEGF in the Chinese Pharmacopoeia, the method has the advantages that: the method is more convenient to realize and the quality is more controllable.
Owner:BIOTECH PHARMA CO LTD
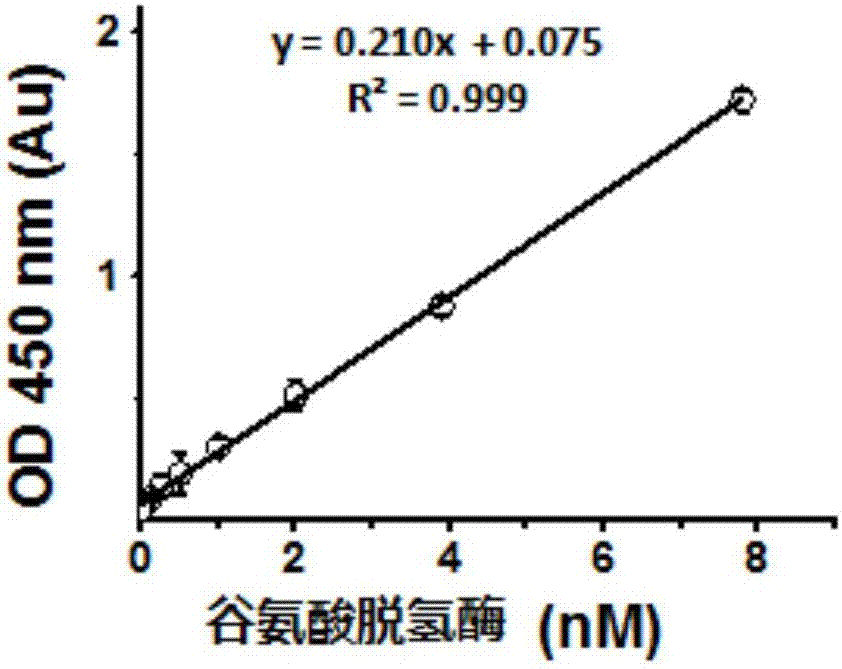
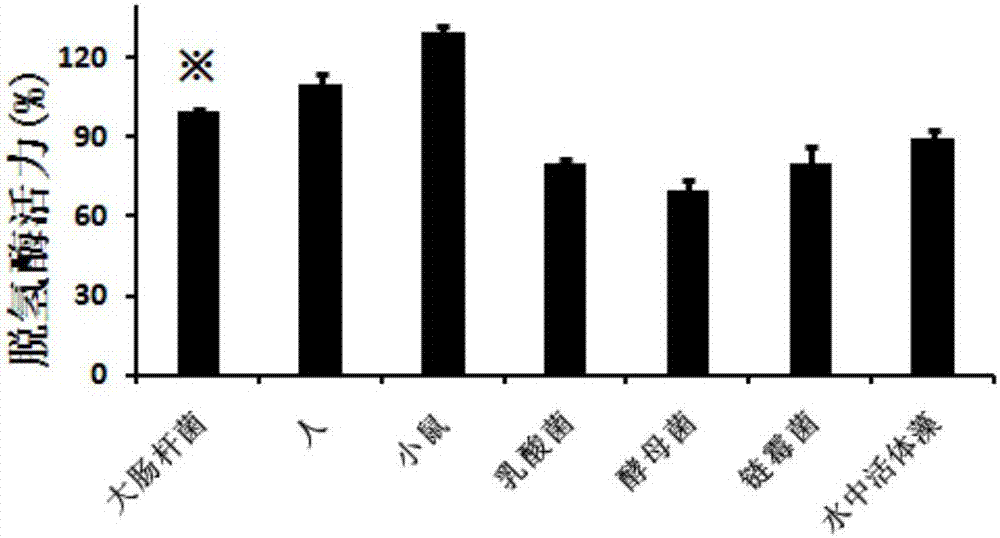
Oxidoreductase activity testing method
The invention relates to the technical field of medicine biological detection, and provides a dehydrogenase activity detection method which can monitor oxidoreductase activity in real time in accordance with color change of a novel tetrazole monosulfonate detection reagent. A tetrazole monosulfonate detection system is adopted, and a good linear dose relationship is presented in detection of dehydrogenase activity; and an EC50 curve is obtained in accordance with an absorbance value of a reactant at 450nm, and dehydrogenase activity evaluation is implemented. The dehydrogenase activity detection method provided by the invention is highly sensitive and is convenient to operate; and the detection method is broad in application range, covering dehydrogenase of various organisms, including archaebacteria (extreme thermophilic bacteria and extreme halophilic bacteria), bacteria (lactobacillus, nitrifying bacteria, escherichia coli, diplococcus pneumoniae and the like), various cellular structure bio-actinomycetes, cyanobacteria (oscillatoria, chroococcus, nostoc and the like), most unicellular algae (chlamydomonas, green algae and the like), fungi (edible fungi, saccharomycetes, mould and the like) as well as animals and plants.
Owner:HANGZHOU JENNIFER BIOTECH CO LTD
Method for detecting cytotoxicity of nano materials through yeast mutant strains
InactiveCN104099397AIncreased sensitivityFaster and more sensitive detectionMicrobiological testing/measurementMicroorganism based processesCytotoxicityOrganism
The invention discloses a method for detecting cytotoxicity of nano materials through yeast mutant strains. The method comprises the following steps: A, knocking out yeast specific genes: extracting saccharomyces cerevisiae genomes, building gene knockout components, transferring the gene knockout components into yeast cells through a lithium acetate conversion method and screening so as to obtain gene knockout yeast; B, preparing nano material suspension liquid; C, testing nano material through yeast; D, detecting concentration of cells after test through yeast; E, computing the result and analyzing: deducting blank, and computing the growth inhibition ratio / cell viability; F, according to the growth inhibition ratio of nano materials to yeast, obtaining half effective concentration EC50 of nano materials to yeast, and then judging toxicity of nano materials. The method is feasible, the operation is simple and convenient, the detection speed is high, the sensitivity is high, the cost is low, the application range is wide, and the method can be used for detecting cytotoxicity of nano materials to organisms in different aqueous solutions.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Agonistic antibody directed against human thrombopoietin receptor
The present invention discloses an agonistic antibody directed against human thrombopoietin receptor (also referred to as 'human c-Mpl). Specifically, the antibody has a constant region having a set of amino acid sequences selected from the following items (1) to (3): (1) amino acid sequences for a heavy-chain constant region and a light-chain constant region of a human antibody; (2) an amino acid sequence for a human antibody heavy-chain constant region in which the domain is replaced by one of other human antibody subclass and an amino acid sequence for a human antibody light-chain constant region; and (3) a set of amino acid sequences having the deletion, substitution, addition or insertion of one or several amino acid residues in each of the amino acid sequences shown in (1) and (2), and the antibody has a variable region capable of binding to a human thrombopoietin receptor to activate the receptor. The antibody also has the following properties: (a) the antibody can induce the formation of a colony at a concentration of 10,000 ng / mL or less in the CFU-MK colony formation assay using a human umbilical cord blood CD34+ cell; and (b) the antibody has the maximum activity higher than that of PEG-rHuMGDF by 50% or more and a 50% effective concentration (EC50) of 100 nM or less in the cell growth assay using an UT7 / TPO cell. Also disclosed is a pharmaceutical composition for the treatment of thrombocytopenia, which comprises the antibody.
Owner:KIRIN PHARMA
Detection of oral microbial virulence factors
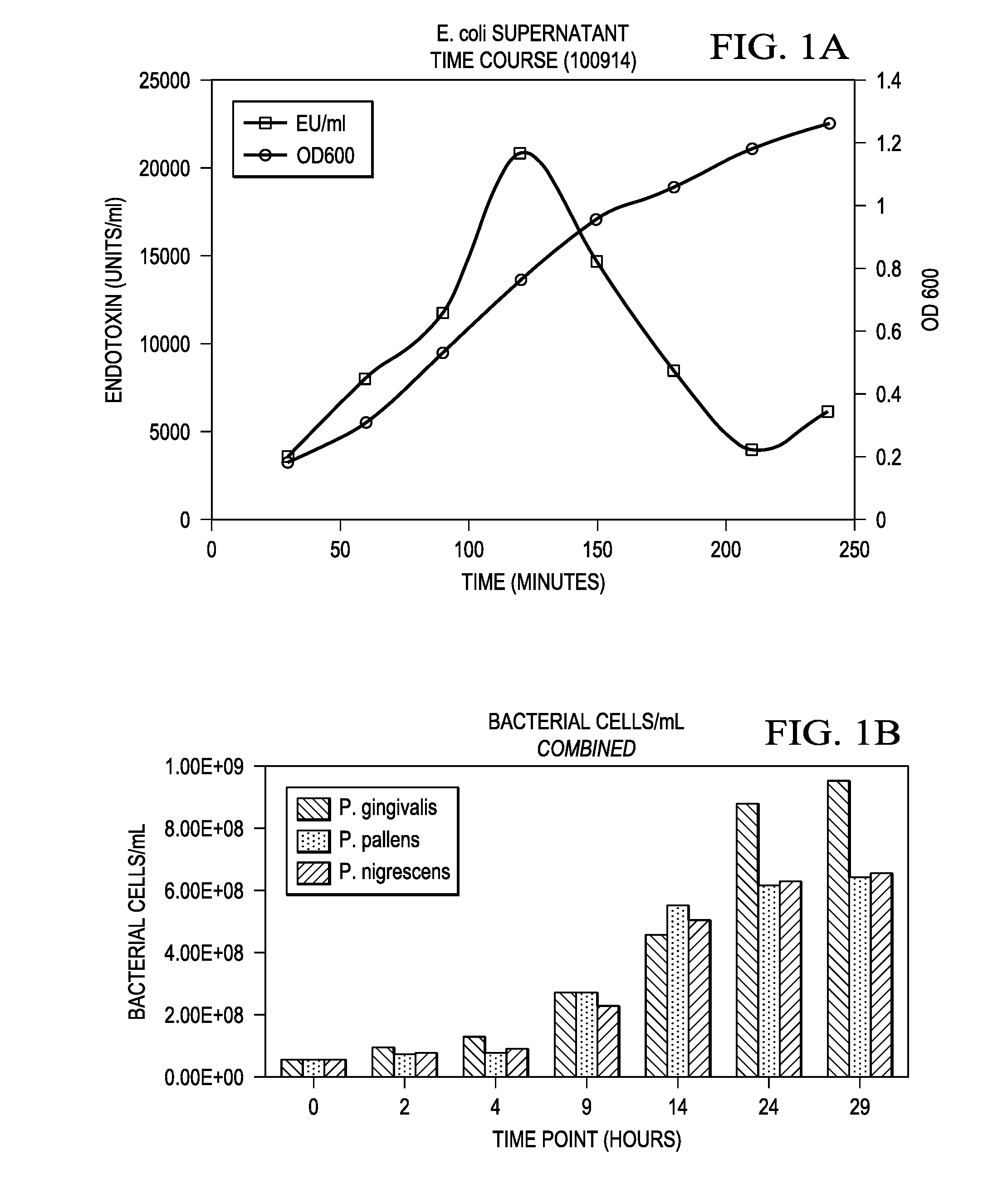
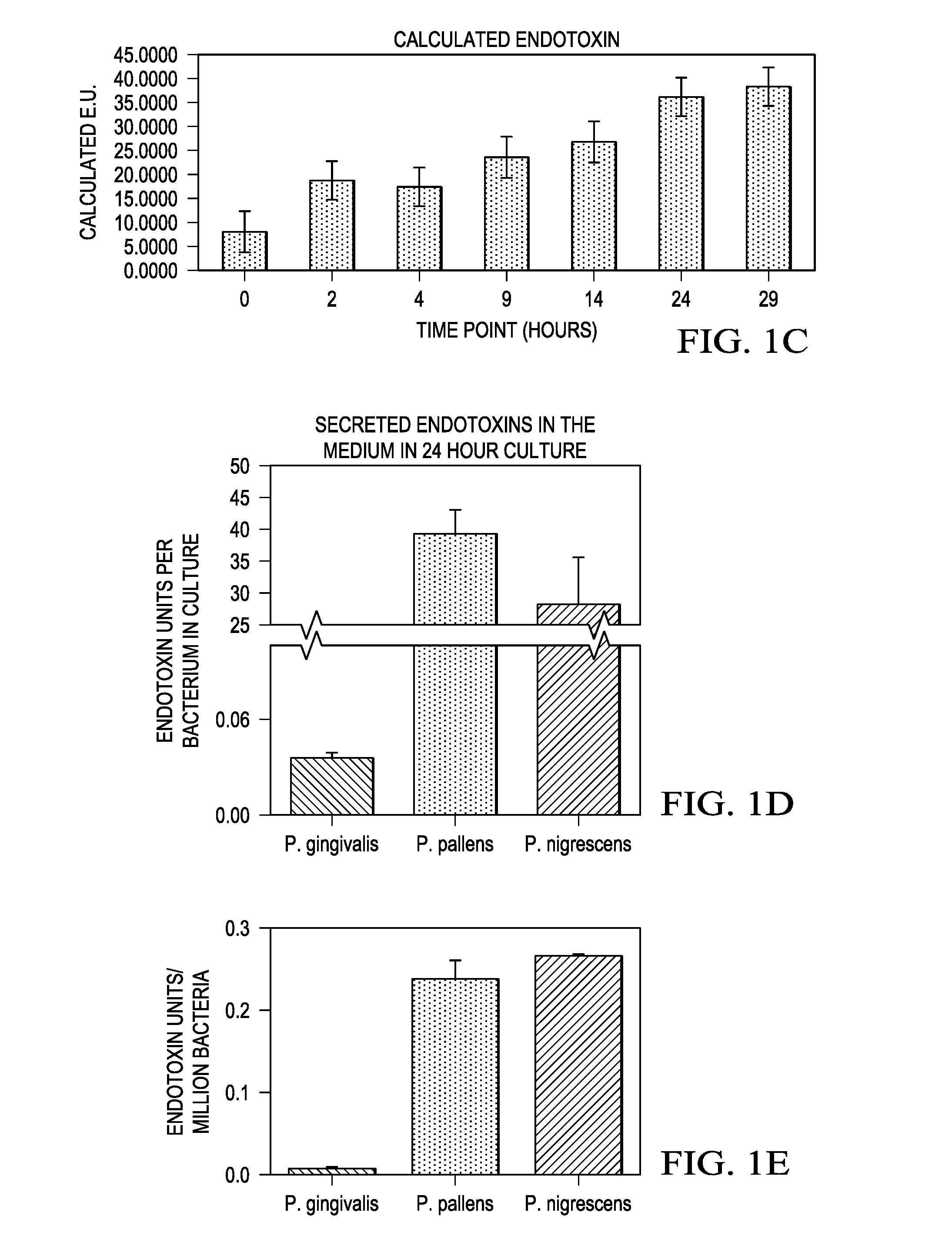
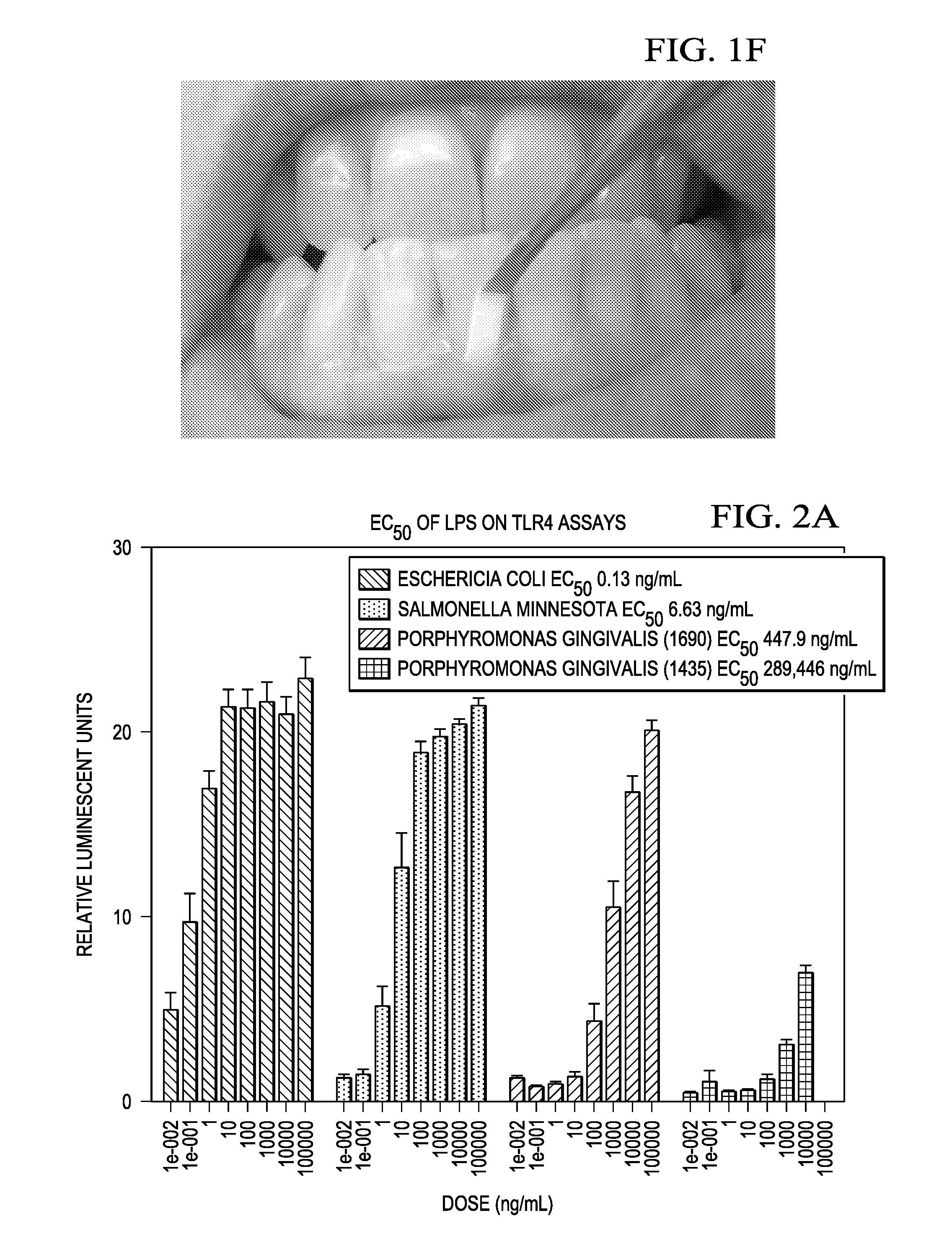
InactiveUS20160327557A1Cosmetic preparationsMicrobiological testing/measurementMicroorganismVirulent characteristics
Methods for detecting and quantifying toxins present in the oral cavity. The methods may include providing a biological sample, providing reporter cells expressing one or more Toll like receptors, exposing the cells to the biological sample, measuring the EC50 value of the lipopolysaccharide on activation of a Toll like receptor, quantification of the lipopolysaccharide in the biological sample.
Owner:THE PROCTER & GAMBLE COMPANY
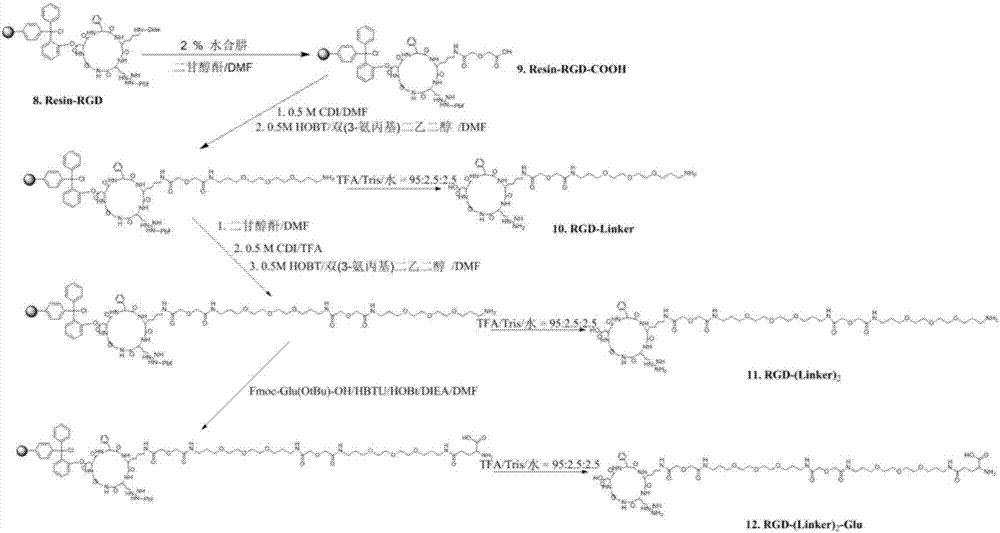
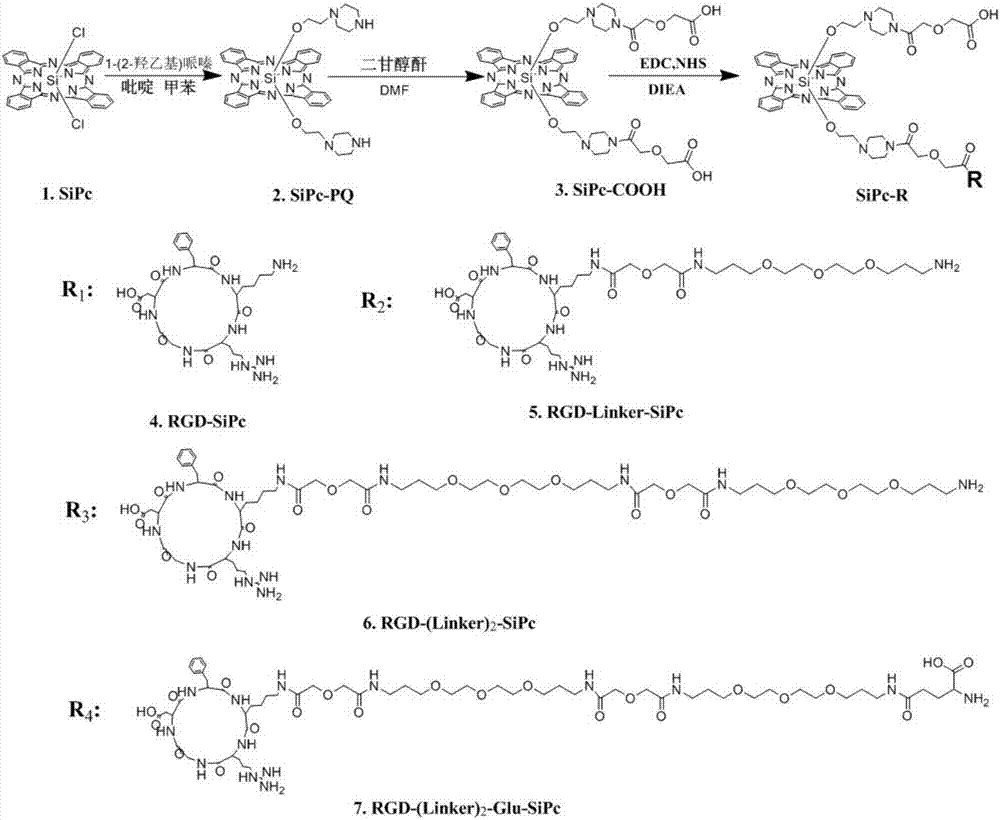
Synthesis and application of RGD polypeptide coupled SiPc (silicon phthalocyanine) photosensitizer
ActiveCN107226839AGood water solubilityResolve aggregationEnergy modified materialsPeptide preparation methodsTumor targetTumor targeting
The invention relates to a SiPc (silicon phthalocyanine) photosensitizer using RGD polypeptide as a targeted group, a preparation method of the SiPc photosensitizer and application of the SiPc photosensitizer to an aspect of tumor photodynamics therapy. The SiPc is used as a photosensitive part to be connected with an RGD polypeptide ligand; PEG (polyethylene glycol) and fragments containing carboxy groups are introduced into the structure; a series of novel photosensitizers with the tumor targeting performance can be prepared. One coupling compound RGD-(Linker)2-Glue-SiPc has good optical physic and photodynamic performance; the EC50 value on the receptor positive tumor cells is 10 to 20nm; under the condition of once medication, the spongioblastoma on the mouse body can be cured; after the continuous observation for 35 days, recurrence does not occur; good application prospects are shown in the field of tumor photodynamics therapy.
Owner:KANGHONG YAOYUAN TECH CO LTD TIANJIN
Litsea cubeba essential oil and preparation method and application thereof
InactiveCN108624406ALower requirementSimple extraction methodEssential-oils/perfumesPreservativeMinimum inhibitory concentration
The invention provides litsea cubeba essential oil and a preparation method and application thereof. The preparation method has the advantages that the litsea cubeba essential oil is extracted from specific litsea cubeba strains, the fact that essential oil content in fresh fruits reaches 6.45% is discovered, geranial content in the essential oil is larger than 48.00%, neral content in the essential oil is larger than 40.00%, limonene content in the essential oil is larger than 3.00%, terpilenol content in the essential oil is larger than 0.30%, and the minimum inhibitory concentration MIC ofthe obtained essential oil is 0.63 microliter / mL which is evidently lower than polyoxins MIC (17.5 microliter / mL); the half-maximum effect concentration EC50 of the essential oil is 0.09 microliter / mLwhich is evidently lower than polyoxins EC50 (1.64 microliter / mL), and the antibacterial effect of the obtained essential oil is evidently higher than that of other litsea cubeba essential oil. The prepared litsea cubeba essential oil can be used for preparing food flavor enhancers, preservatives and additives and has promising market application prospect in fields of food, medicine and daily chemical products.
Owner:RES INST OF SUBTROPICAL FORESTRY CHINESE ACAD OF FORESTRY
Electrophysiological patch clamp local perfusion liquid replacing and feeding head
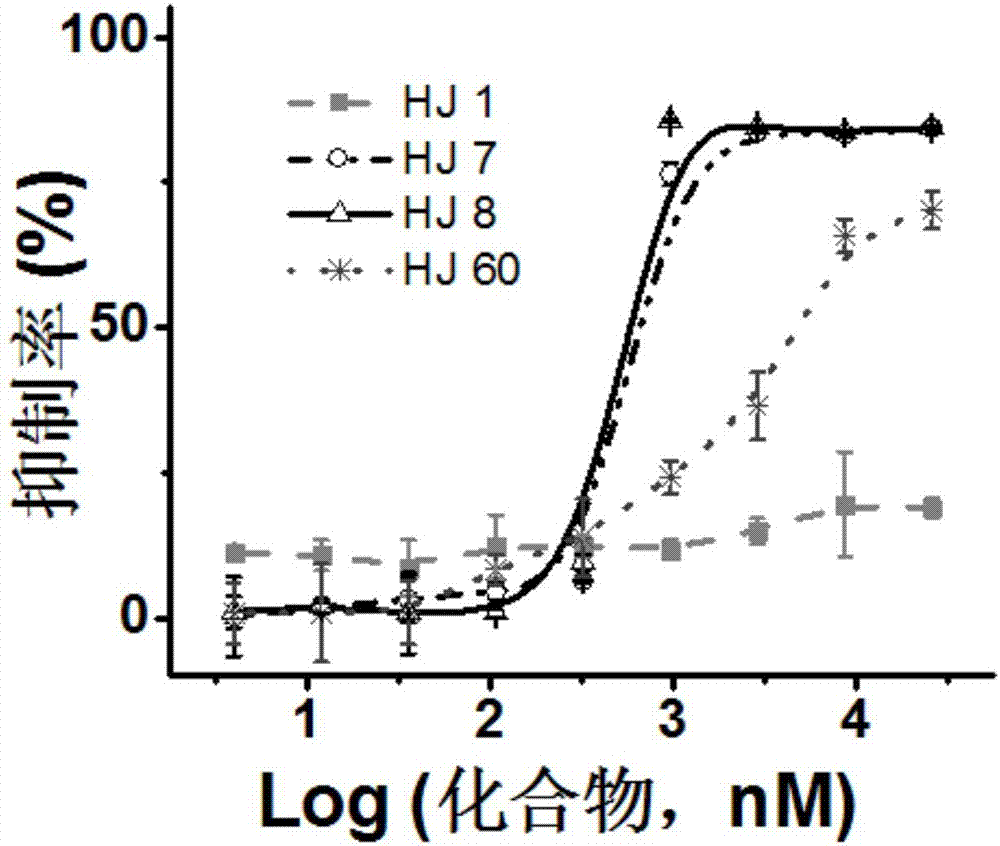
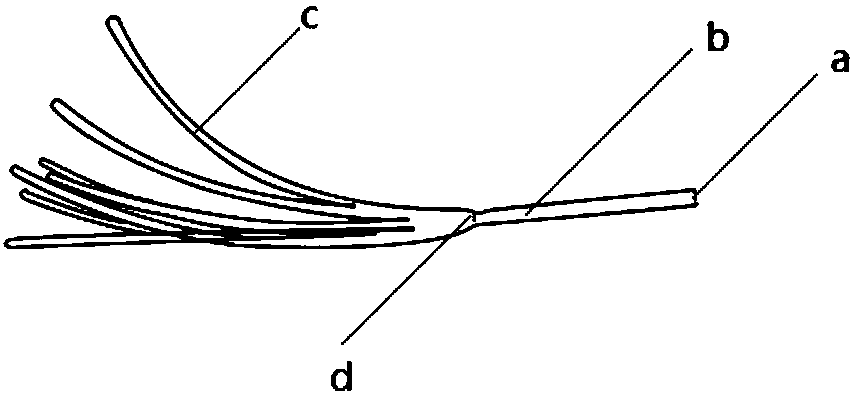
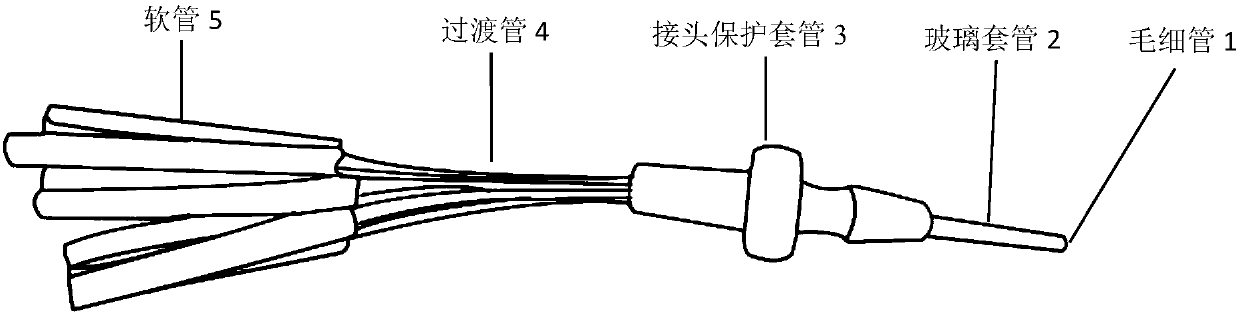
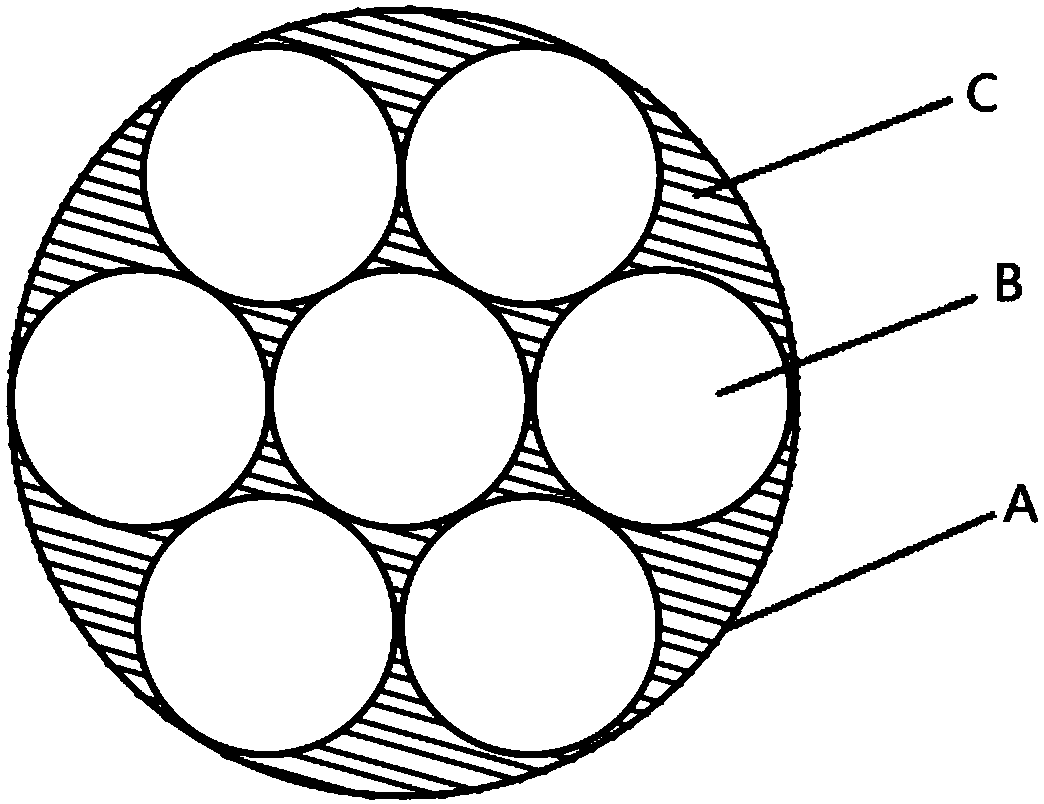
The invention provides an electrophysiological patch clamp local perfusion liquid replacing and feeding head, which comprises a plurality of capillary tubes, a glass bushing, a joint protection bushing, a plurality of transition tubes and a plurality of hoses, wherein the numbers of the capillary tubes, the transition tubes and the hoses are the same, one ends of each capillary tube are respectively connected to one ends of the transition tube, the glass bushing is sleeved outside the capillary tube bundle, the end surface of one end, exposed to the glass bushing, of the capillary tube is a flatly-polished and gap-free plane, the joint protection bushing is sleeved outside the joint position of the capillary tube and the transition tube, and one ends of each hose are respectively connectedto one ends of each transition tube. According to the present invention, the electrophysiological patch clamp local perfusion liquid replacing and feeding head has advantages of firmness, durability,convenient operation, multi-channel medicine input, single-channel medicine output, stable flow rate of medicine, low feeding error, high accuracy, low requirements on the properties of medicine, acid resistance, corrosion resistance, high-temperature resistance, wide application range, intuitive liquid feeding, gas bubble generation reducing, experimental error reducing, accurate experimental result and the like, and can be used for determining concentration curves and other kinetic parameters and determine the EC50, the IC50 and the like of medicine.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com